Question: Given the following data for the following half-reactions and E degree (V vs. NHE): Cu2 + 2e= Cu E=0.340V Cu* + + e=Cul E=0.86V Oz
Given the following data for the following half-reactions and E degree (V vs. NHE):
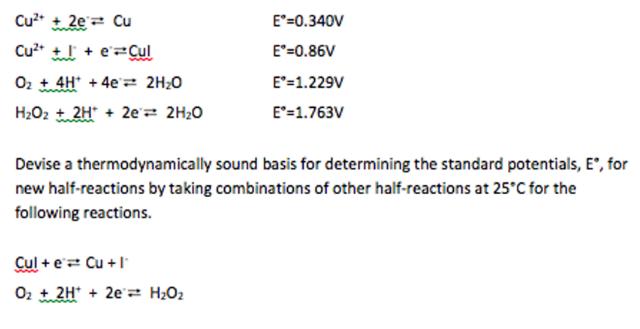
Cu2 + 2e= Cu E=0.340V Cu* + + e=Cul E"=0.86V Oz t4H + 4e 2H;0 E'=1.229V H2O2 t 2H" + 2e'2 2H20 E'=1.763V Devise a thermodynamically sound basis for determining the standard potentials, E', for new half-reactions by taking combinations of other half-reactions at 25C for the following reactions. Cul + e Cu + I Oz + 2H* + 2e= H2O2
Step by Step Solution
There are 3 Steps involved in it
To find the standard potentials Ecirc for the new halfreactions we need to use the given standard po... View full answer

Get step-by-step solutions from verified subject matter experts


