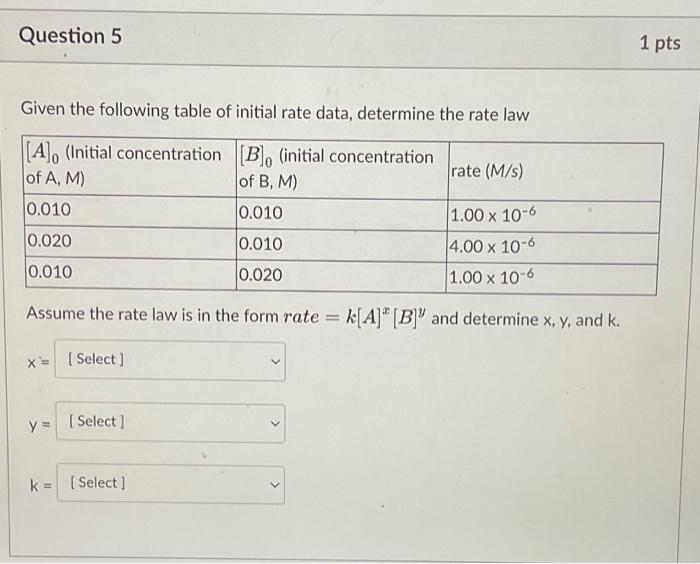
Question: Given the following table of initial rate data, determine the rate law Assume the rate law is in the form rate =k[A]x[B]y and determine x,y,
![law Assume the rate law is in the form rate =k[A]x[B]y and](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f90cbb8b018_75566f90cbb2c3e3.jpg)

Given the following table of initial rate data, determine the rate law Assume the rate law is in the form rate =k[A]x[B]y and determine x,y, and k. x= y= k= For the reaction: A+B+Cproducts With the rate law: rate=k[A][B]2 Complete the following statements regarding reaction order: The reaction is order in A. The reaction is order in B. The reaction is order in C. The reaction is order overall. Which of the following effect rates of chemical reactions? Select all that apply. Temperature Concentration Presence of a catalyst Physical state of the reactants Month of the year
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


