Question: Given the reactioin below, what products would you expect for this reaction? (Hint: Draw your dot structure for the compound. Notice how nitrogen has three
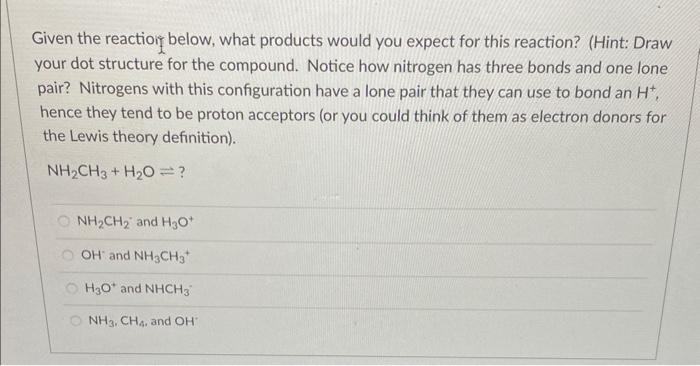
Given the reactioin below, what products would you expect for this reaction? (Hint: Draw your dot structure for the compound. Notice how nitrogen has three bonds and one lone pair? Nitrogens with this configuration have a lone pair that they can use to bond an H+, hence they tend to be proton acceptors (or you could think of them as electron donors for the Lewis theory definition). NH2CH3+H2O? NH2CH2+and H3O+ OHand NH3CH3+ H3O+and NHCH3 NH3,CH4, and OH
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


