Question: Given these reactions, where X represents a generic metal or metalloid 1) H,(g) + O2(g) HO(g) AH = -241.8 kJ 2) X(s) + 2Cl2(g) XCI,
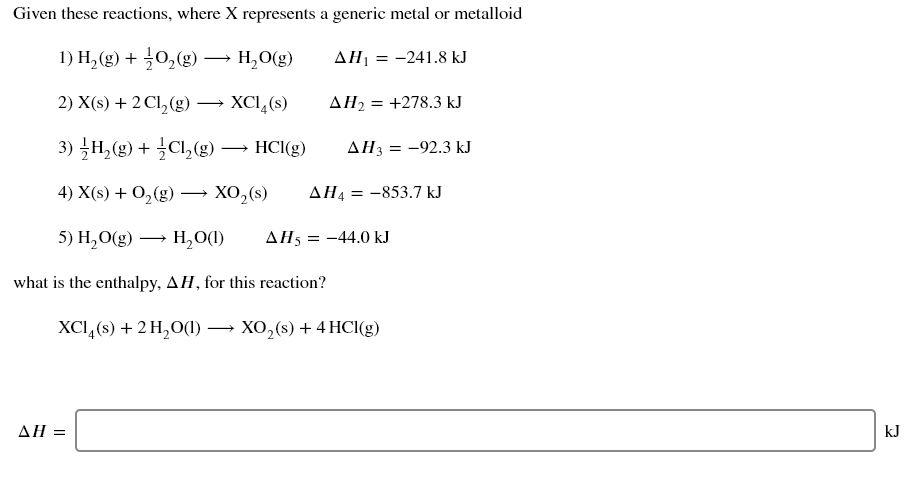
Given these reactions, where X represents a generic metal or metalloid 1) H,(g) + O2(g) HO(g) AH = -241.8 kJ 2) X(s) + 2Cl2(g) XCI, (S) AH2 = +278.3 kJ 3) H2(g) + 2Cl2(8) HCI(g) AH3 = -92.3 kJ 4) X(s) + O2(g) XO (S) AHA = -853.7 kJ 5) H2O(g) H0(1) AH = -44.0 kJ what is the enthalpy, AH, for this reaction? XCI,(s) + 2 H2O(l) XO,() + 4 HCl(g) AH = kJ
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


