Question: Explain the values of the kinetic isotope effect (kH/kD) for the following reactions, indicating what each value means, the reason for the determination made, and
Explain the values of the kinetic isotope effect (kH/kD) for the following reactions, indicating what each value means, the reason for the determination made, and the mechanism of each reaction that supports your answer.
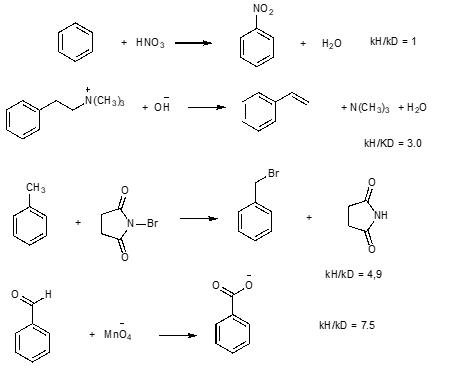
+ HNO3 - 8 N(CH3) CH3 N-Br + OH + MnO4 * NO + 8 HO KH/KD = 1 + N(CH3)3 + HO kH/KD = 3.0 = Br 6. & NH kH/KD = 4,9 KH/KD = 7.5
Step by Step Solution
3.38 Rating (160 Votes )
There are 3 Steps involved in it
mechanism H Reaction1 H 2 HNO 44 ... View full answer

Get step-by-step solutions from verified subject matter experts


