Question: - Gray =C; white =H; red =O; blue =N; dark green =Cl; brown =Br; light green =F; purple =I; yellow =S; orange =P. Use your
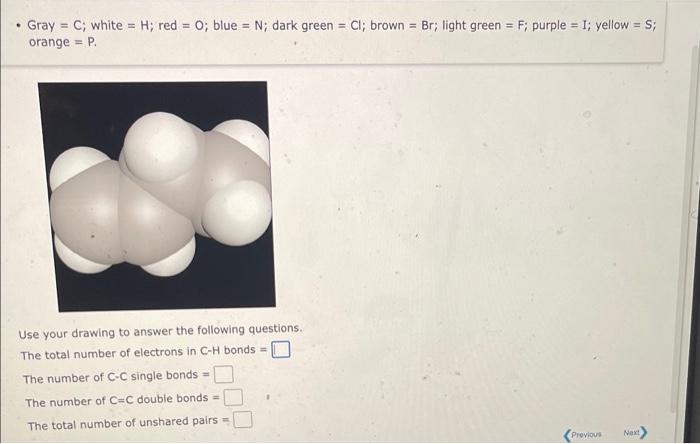
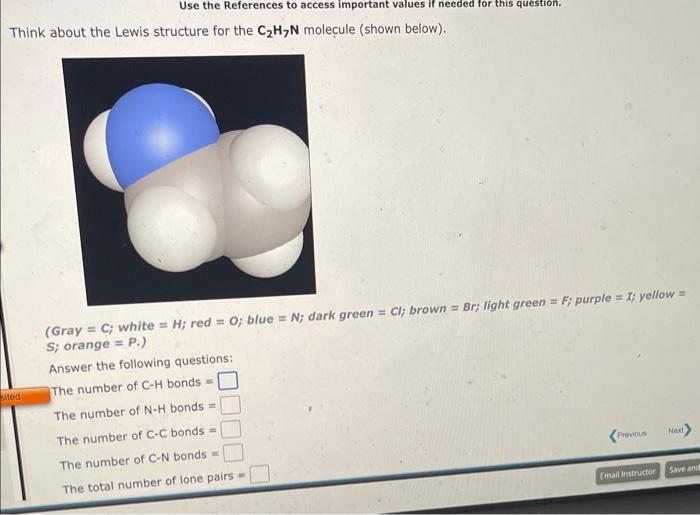
- Gray =C; white =H; red =O; blue =N; dark green =Cl; brown =Br; light green =F; purple =I; yellow =S; orange =P. Use your drawing to answer the following questions. The total number of electrons in CH bonds = The number of CC single bonds = The number of C=C double bonds = The total number of unshared pairs = Use the References to access important values if needed for this question. Think about the Lewis structure for the C2H7N molecule (shown below). (Gray =C; white =H; red =O; blue =N; dark green =Cl; brown = Br; light green = F; purple =; yellow = S; orange = P. ) Answer the following questions: The number of CH bonds = The number of NH bonds = The number of CC bonds = The number of CN bonds = The total number of lone pairs =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


