Question: Think about the Lewis structure for the CH2O2 molecule (shown below). (Gray =C; white =H; red =O; blue =N; dark green =Cl; brown =Br; light
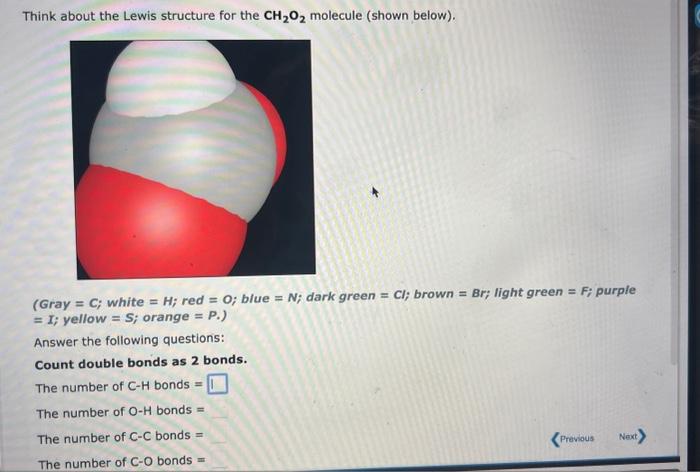
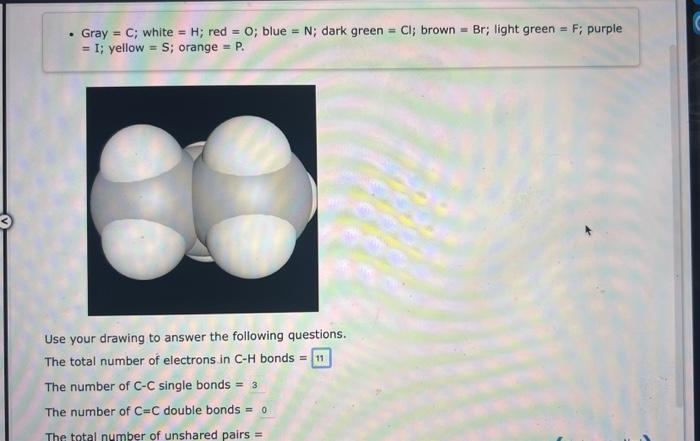
Think about the Lewis structure for the CH2O2 molecule (shown below). (Gray =C; white =H; red =O; blue =N; dark green =Cl; brown =Br; light green =F; purple =I; yellow =S; orange =P.) Answer the following questions: Count double bonds as 2 bonds. The number of CH bonds = The number of OH bonds = The number of C - C bonds = The number of CO bonds = - Gray =C; white =H; red =O; blue =N; dark green =Cl; brown =Br; light green =F; purple =I; yellow =S; orange =P. Use your drawing to answer the following questions. The total number of electrons in CH bonds = The number of CC single bonds =3 The number of C=C double bonds =0 The total number of unshared pairs = The element silicon would be expected to form covalent bond(s) in order to obey the octet rule. Use the octet rule to predict the formula of the compound that would form between silicon and hydrogen, if the molecule contains only one silicon atom and only single bonds are formed. Formula
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


