Question: Hello , i need help on this exercise i would appreciate if you explained the process of solving it and where to get the values
Hello , i need help on this exercise i would appreciate if you explained the process of solving it and where to get the values !!
For a flash distillation process, we have the following data and results:
xF= 0.3 f= 0.4 xB=0.45 yD= 0.66 Tcalc.= 93.C
The enthalpy of evaporation and the specific heats of liquids are:
for benzene: = 7, 360 cal/g mol, Cp = 33 cal/mol C
for toluene: = 7,960 cal/g mol, Cp = 40 cal/mol C? )
Remember:
Cp avg (feed)= XF(Cp benc) + (1-XF) Cp Tol
avg = YD(benc) + (1-YD)(tol)
The enthalpy balance produces the following equation:
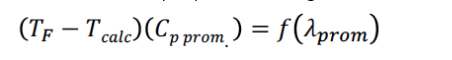
Using the above information, calculate: (Take all answers to two decimal places)
Cp avg= _____ avg = _______
T F= _______C
(TFTcalc)(Cpprom.)=f(prom)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


