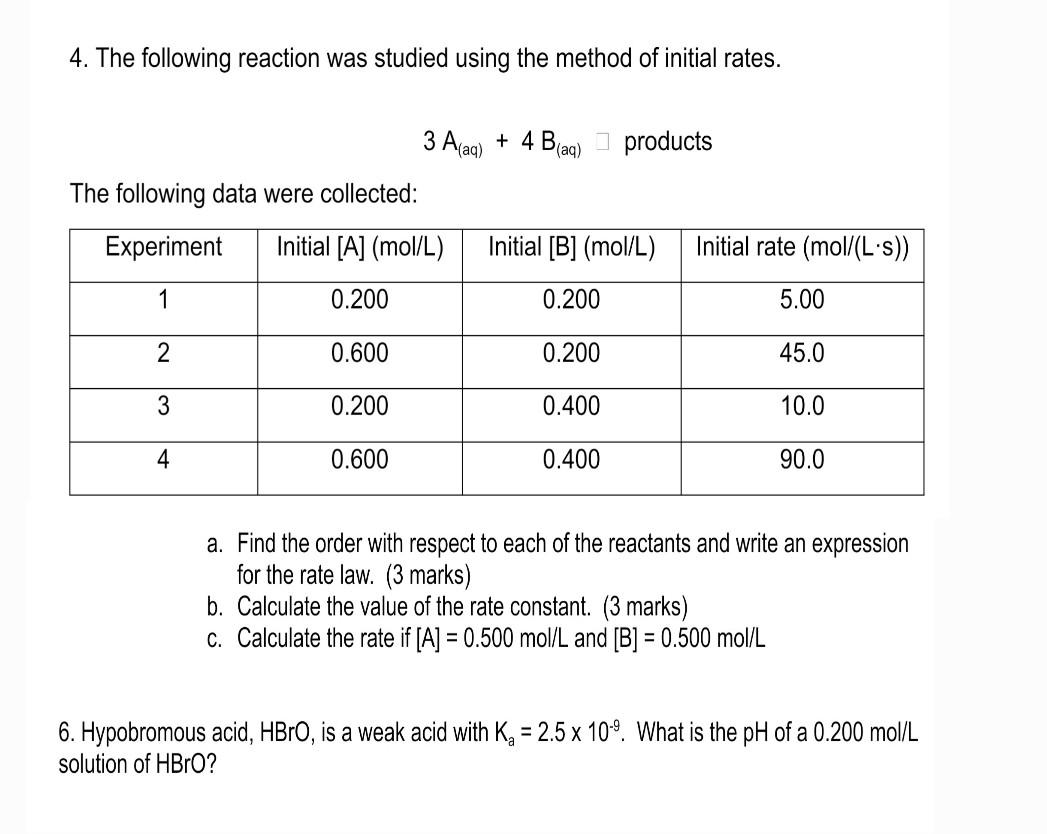
Question: Hello Please help me with both 2 and 3 4. The following reaction was studied using the method of initial rates. 3A(aq)+4B(aq)]products The following data
Hello Please help me with both 2 and 3
4. The following reaction was studied using the method of initial rates. 3A(aq)+4B(aq)]products The following data were collected: a. Find the order with respect to each of the reactants and write an expression for the rate law. (3 marks) b. Calculate the value of the rate constant. ( 3 marks) c. Calculate the rate if [A]=0.500mol/L and [B]=0.500mol/L 6. Hypobromous acid, HBrO, is a weak acid with Ka=2.5109. What is the pH of a 0.200mol/L solution of HBrO
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


