Question: hey please help me with this question 2. The following reaction was studied using the method of initial rates. 3A(aq)+4B(aq)products The following data were collected:
hey please help me with this question
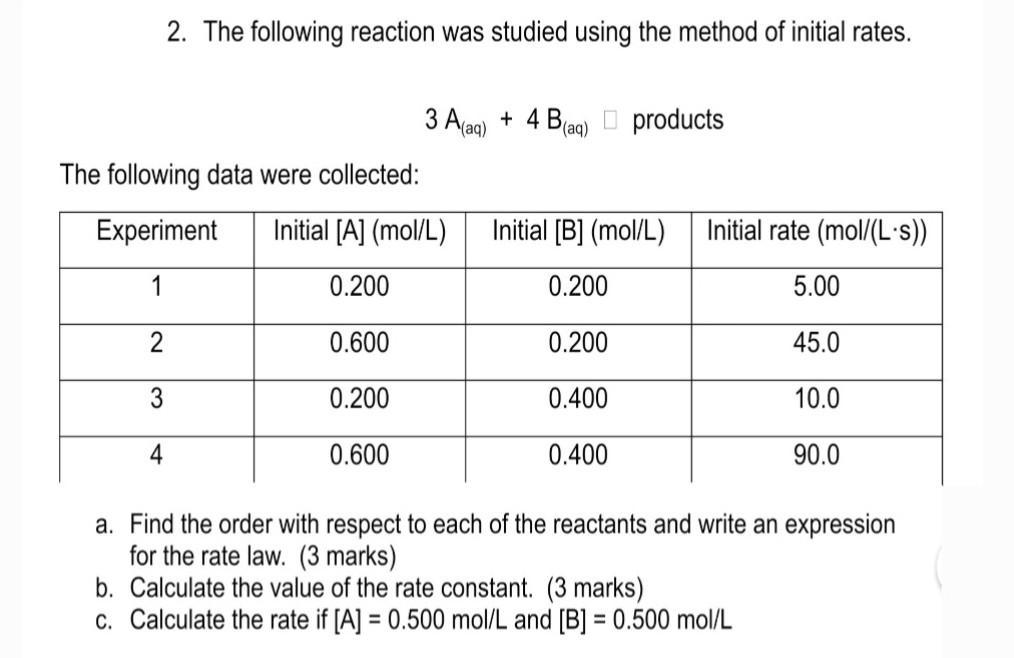
2. The following reaction was studied using the method of initial rates. 3A(aq)+4B(aq)products The following data were collected: a. Find the order with respect to each of the reactants and write an expression for the rate law. (3 marks) b. Calculate the value of the rate constant. ( 3 marks) c. Calculate the rate if [A]=0.500mol/L and [B]=0.500mol/L
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


