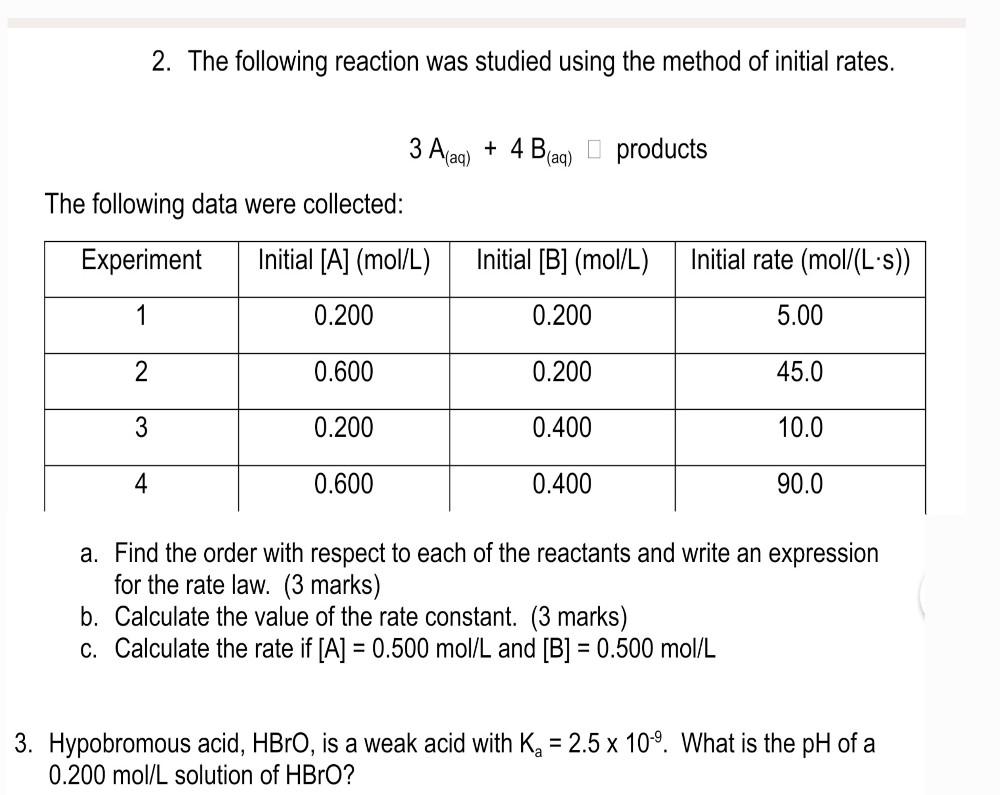
Question: please help me with both 2 and 3c 2. The following reaction was studied using the method of initial rates. 3A(aq)+4B(aq)products The following data were
please help me with both 2 and 3c
2. The following reaction was studied using the method of initial rates. 3A(aq)+4B(aq)products The following data were collected: a. Find the order with respect to each of the reactants and write an expression for the rate law. (3 marks) b. Calculate the value of the rate constant. (3 marks) c. Calculate the rate if [A]=0.500mol/L and [B]=0.500mol/L Hypobromous acid, HBrO, is a weak acid with Ka=2.5109. What is the pH of a 0.200mol/L solution of HBrO
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


