Question: help 1 points A student conducted a kinetic study to determine the integrated rate law for a chemical reaction. He found that a graph where
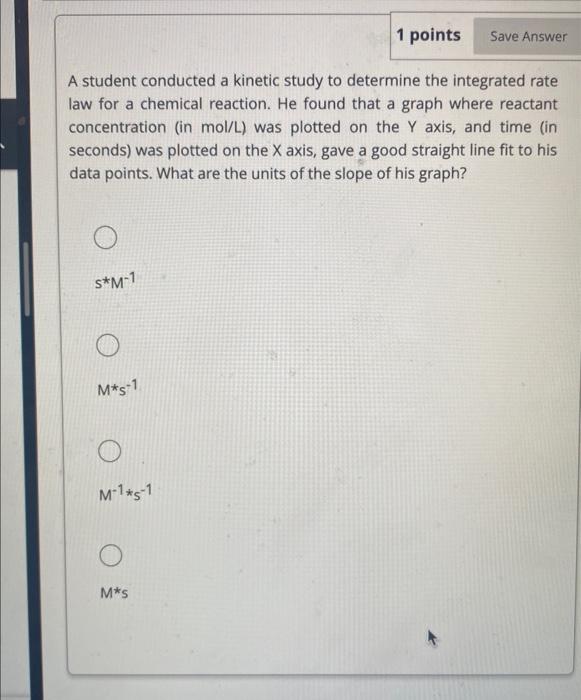
1 points A student conducted a kinetic study to determine the integrated rate law for a chemical reaction. He found that a graph where reactant concentration (in mol/L ) was plotted on the Y axis, and time (in seconds) was plotted on the X axis, gave a good straight line fit to his data points. What are the units of the slope of his graph? sM1 Ms1 M1s1 Ms
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


