Question: help 1/T(K) vs. logPH2O 1. Based on your graph of logPH2O versus 1/T and trendline equation, what is the vapor pressure of water in torr
help
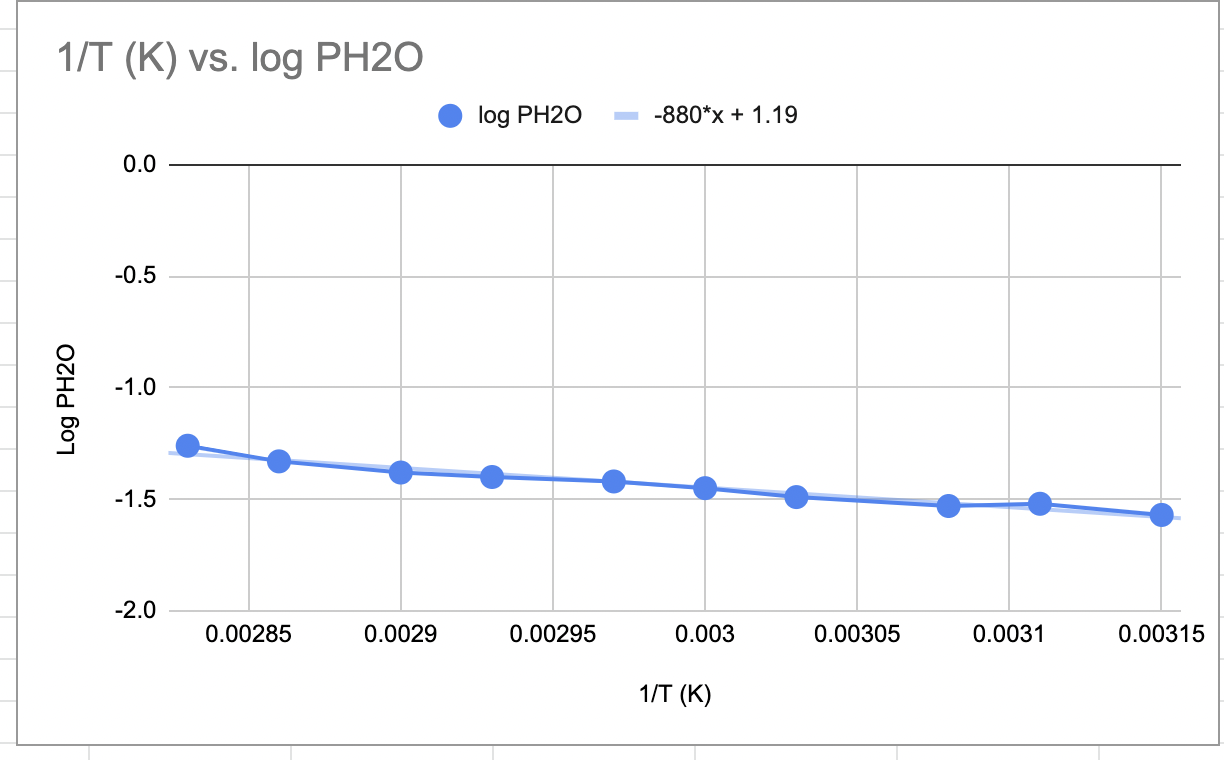

1/T(K) vs. logPH2O 1. Based on your graph of logPH2O versus 1/T and trendline equation, what is the vapor pressure of water in torr at 67C ? 2. Based on the actual value for Hvap of water, how many kJ of energy are needed to convert 5.00 kg of water into steam at 100C ? 3. Diethyl ether (molar mass 74.12g/mol ) boils at 34.6C and water (molar mass 18.02g/mol ) boils at 100C. a. At 25C, which compound - diethyl ether or water - has the higher vapor pressure? b. Which data - molar mass or boiling point - did you use to make your choice? Explain your answer. 4. Why does steam at 100C cause a more severe burn than liquid water at 100C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


