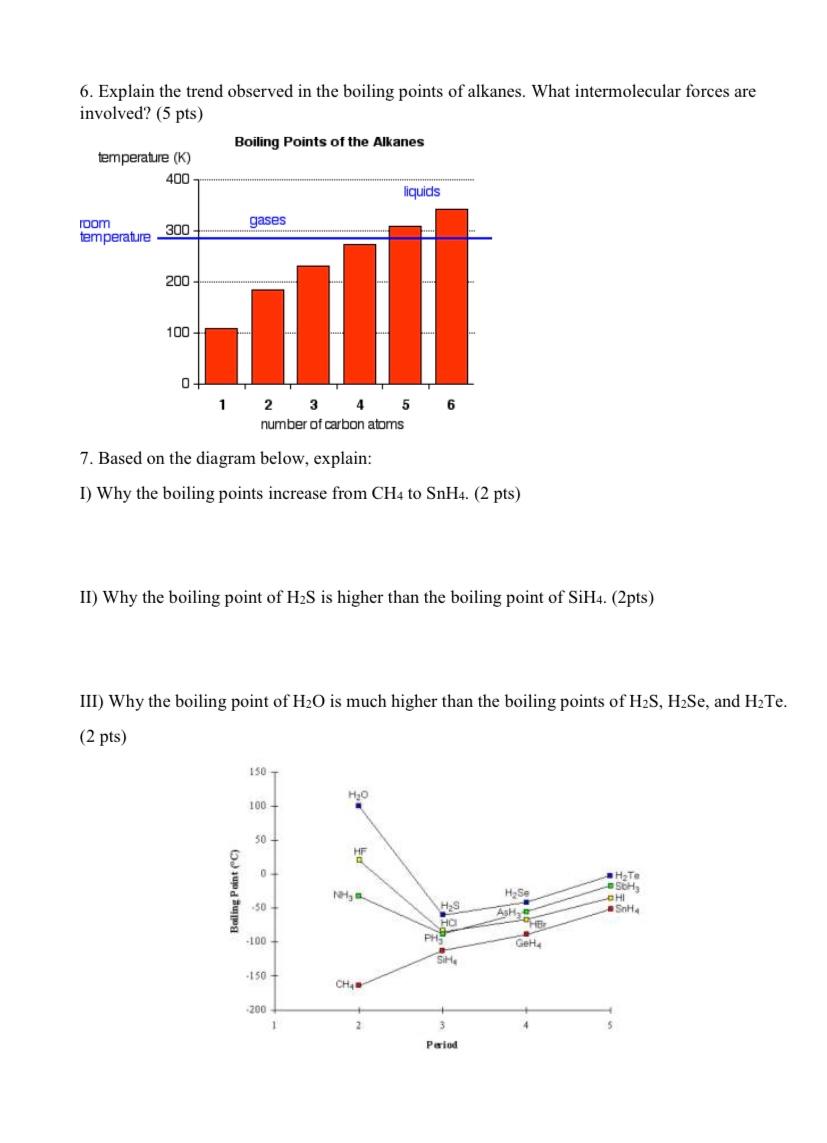
Question: help 6. Explain the trend observed in the boiling points of alkanes. What intermolecular forces are involved? (5 pts) 7. Based on the diagram below,
 help
help
6. Explain the trend observed in the boiling points of alkanes. What intermolecular forces are involved? (5 pts) 7. Based on the diagram below, explain: I) Why the boiling points increase from CH4 to SnH4.(2pts) II) Why the boiling point of H2S is higher than the boiling point of SiH4.(2pts) III) Why the boiling point of H2O is much higher than the boiling points of H2S,H2Se, and H2Te. (2 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


