Question: help answer question #6 using data set for solution A and B please :) Data: 1. Freezing point of distilled water (T.20) 0.3 C 2.
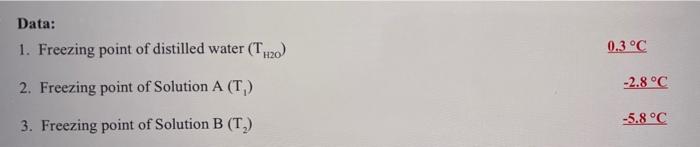

Data: 1. Freezing point of distilled water (T.20) 0.3 C 2. Freezing point of Solution A (T) -2.8C -5.8 C 3. Freezing point of Solution B (T) 6. Calculate molar mass of antifreeze by dividing grams of antifreeze by number of moles of antifreeze in each solution Solution A: Solution B
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


