Question: help asap please i will upvote 1) In this experiment, you will be mixing aqueous solutions of sodium carbonate and calcium chloride to produce solid
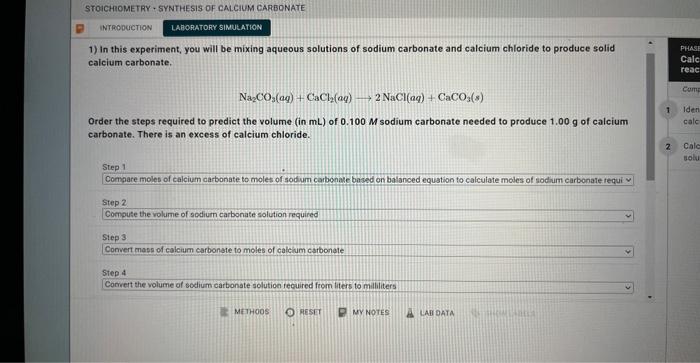
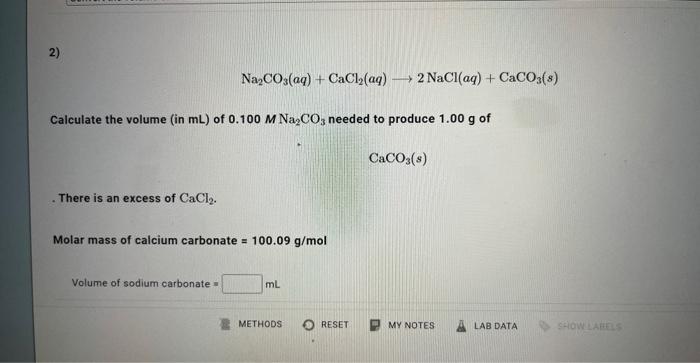
1) In this experiment, you will be mixing aqueous solutions of sodium carbonate and calcium chloride to produce solid calcium carbonate. Na2CO3(aq)+CaCl2(aq)2NaCl(aq)+CaCO3(s) Order the steps required to predict the volume (in mL ) of 0.100M sodium carbonate needed to produce 1.00g of calcium carbonate. There is an excess of calcium chloride. Na2CO3(aq)+CaCl2(aq)2NaCl(aq)+CaCO3(s) Calculate the volume (in mL ) of 0.100MNa2CO3 needed to produce 1.00g of CaCO3(s) There is an excess of CaCl2. Molar mass of calcium carbonate =100.09g/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


