Question: Help Given the following data, determine the orders (m and n) with respect to the concentrations of A and B in the reaction A+BproductsRate=k[A]m[B]n Orders:
Help
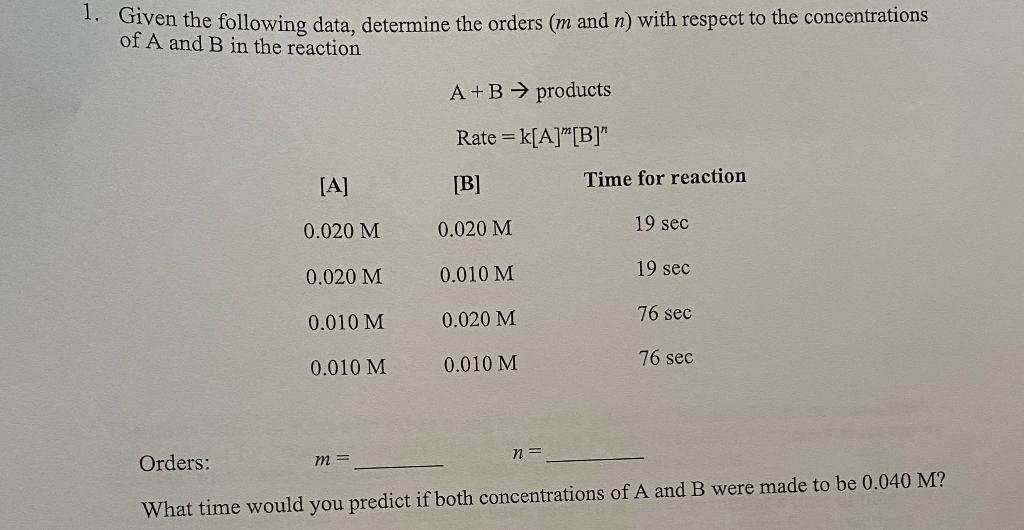
Given the following data, determine the orders (m and n) with respect to the concentrations of A and B in the reaction A+BproductsRate=k[A]m[B]n Orders: m= n= What time would you predict if both concentrations of A and B were made to be 0.040M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


