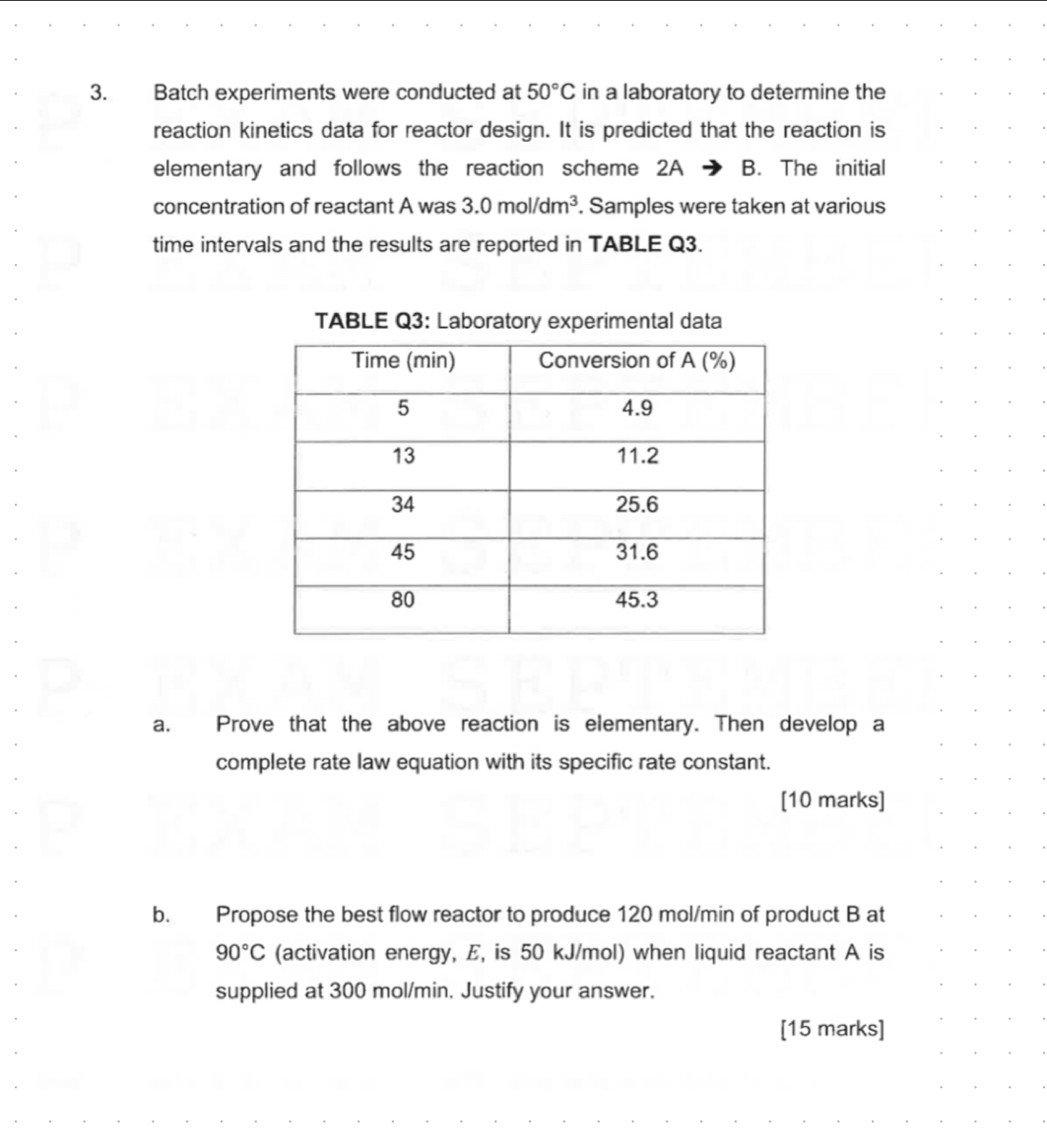
Question: Batch experiments were conducted at 5 0 C in a laboratory to determine the reaction kinetics data for reactor design. It is predicted that the
Batch experiments were conducted at in a laboratory to determine the reaction kinetics data for reactor design. It is predicted that the reaction is elementary and follows the reaction scheme The initial concentration of reactant A was Samples were taken at various time intervals and the results are reported in TABLE Q
TABLE Q: Laboratory experimental data
tableTime minConversion of A
a Prove that the above reaction is elementary. Then develop a complete rate law equation with its specific rate constant.
marks
b Propose the best flow reactor to produce of product at activation energy, is when liquid reactant is supplied at Justify your answer.
marks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


