Question: help i dont understand how to do 3a,b,c PART 3A: Reaction between Magnesium and Hydrochloric Acid Mg(s)+2HCl(aq)MgCl2(aq)+H2(g) Mass of magnesium Moles of magnesium Volume of

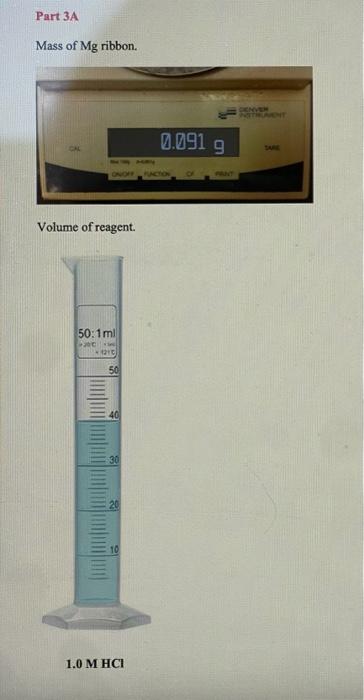
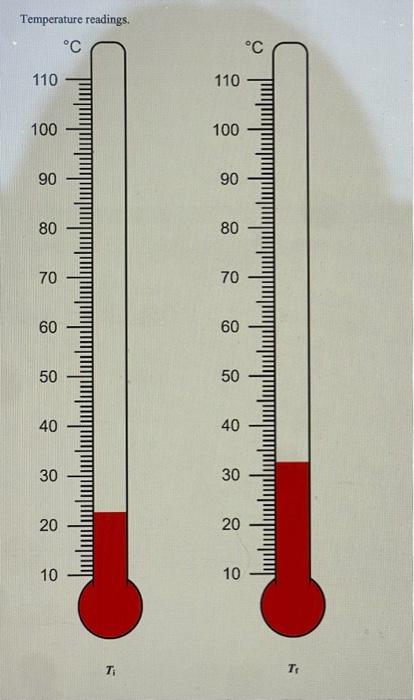

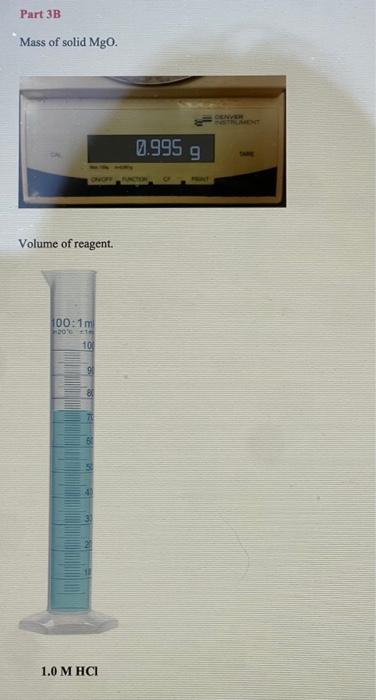
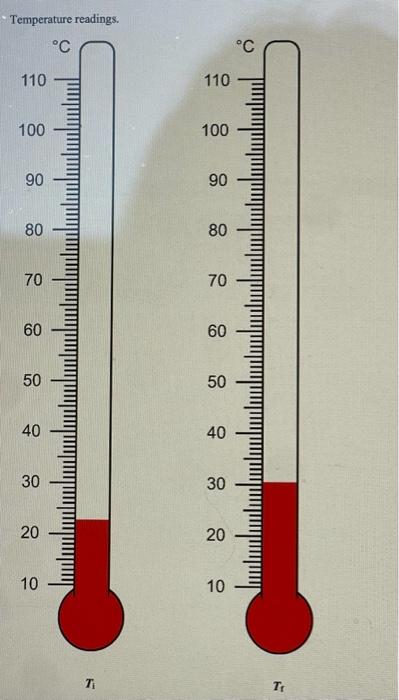

PART 3A: Reaction between Magnesium and Hydrochloric Acid Mg(s)+2HCl(aq)MgCl2(aq)+H2(g) Mass of magnesium Moles of magnesium Volume of 1.0MHCl Moles of HCl Mass of 1.0MHCl solution Mass of 1.0MHCl d=1.017g/mL plus Mg (total mass Initial temperature of HCl Final temperature o Change in temperature Heat of neutralization ( qrnn) cs=4.02J/gC Show work Enthalpy of reaction (Hnn) Determine the limiting reagent and show work Mass of Mg ribbon. Volume of reagent. II OL 0z 0 0t 09 09 0L 08 06 00L OLL D. 's8utpear muexdua L. MgO(s)+2HCl(aq)MgCl2(aq)+H2O Mass of magnesium oxide Moles of magnesium oxide. Volume of 1.0MHCl Moles of HCl Mass of 1.0MHCl Mass of 1.0MHCl solution d=1.017g/mL plus MgO (total mass) Initial temperature of HCl Final temperature of solution Change in temperature Heat of neutralization ( qmnn ) cs=4.02.J/gC Show work Enthalpy of reaction (Hnn) Determine the limiting reagent and show work Mass of solid MgO. Volume of reagent. 1.0MHCl 7 Part 3C: Hess's Law Calculation Use thermochemical chemical Equations 8.5 and 8.6 along with the heat of formation for water: H2(g)+1/2O2(g)H2O(l)Hr=285.8kJ and apply Hess's Law to determine Hf of magnesium oxide Mg(s)+1/2O2(g)MgO(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


