Question: Help me solve these! What is the final volume V2 in milliliters when 0.941 L of a 40.3 % (m/v) solution is diluted to 22.8
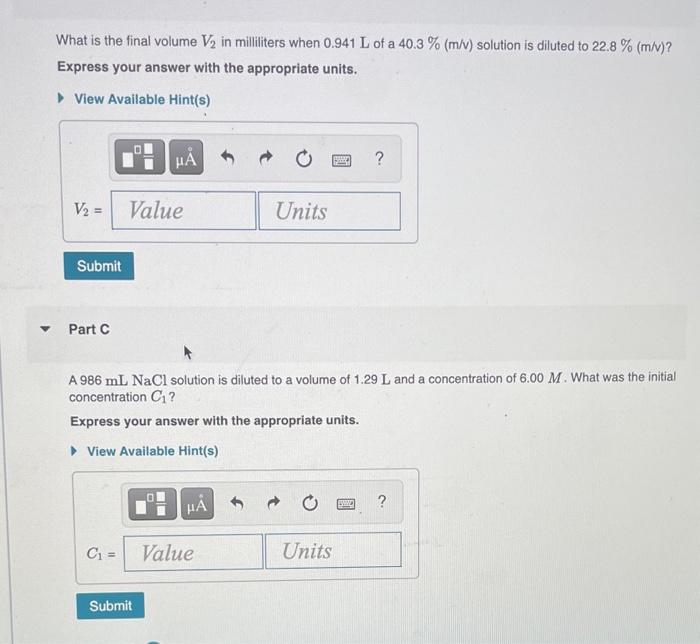
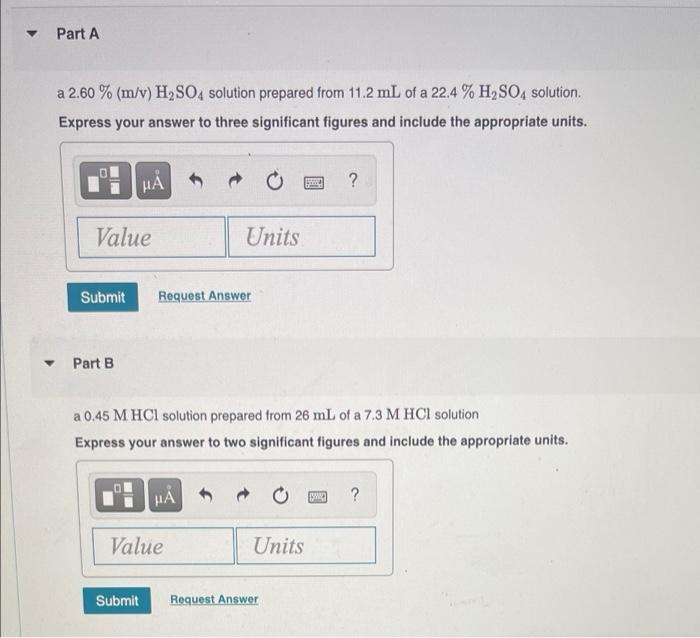
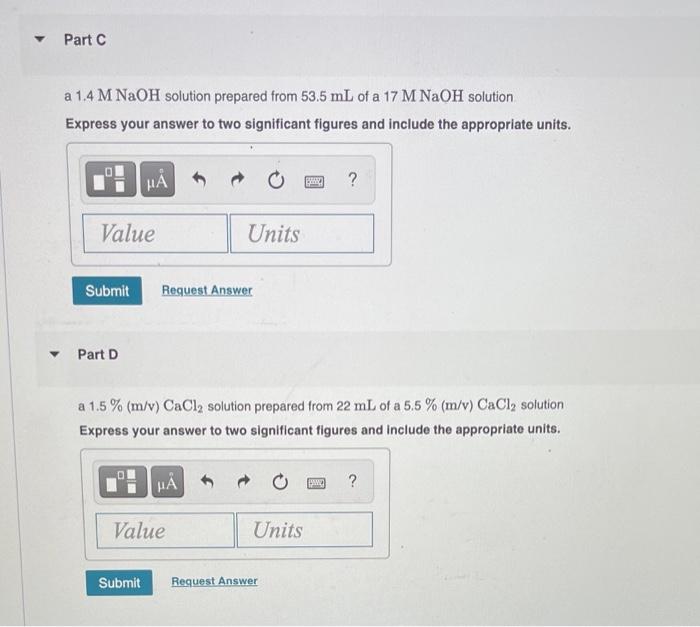
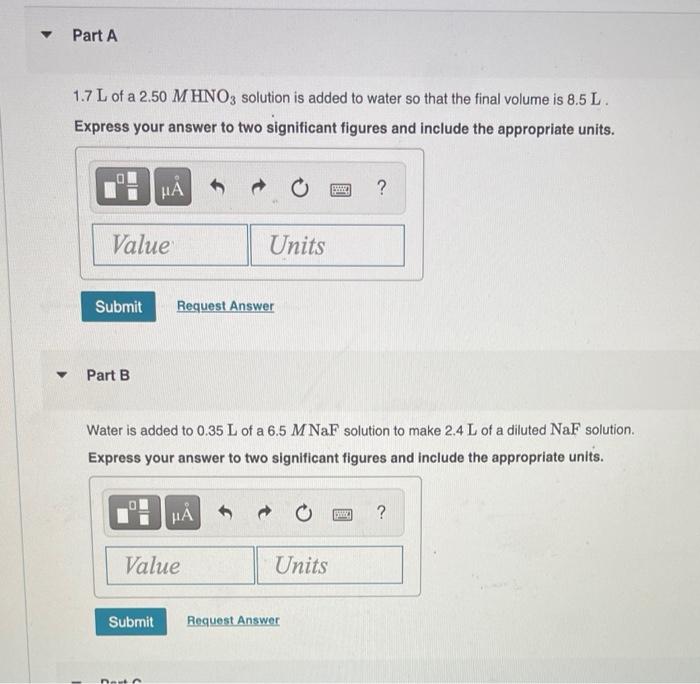
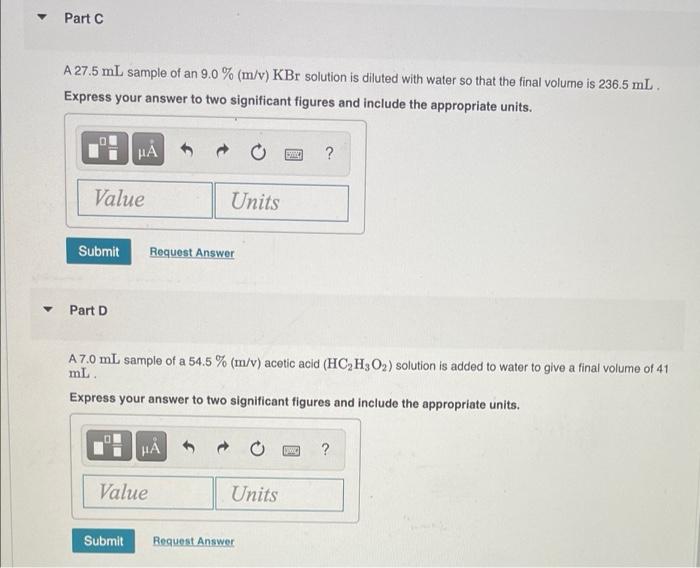
What is the final volume V2 in milliliters when 0.941 L of a 40.3 % (m/v) solution is diluted to 22.8 % (m/v)? Express your answer with the appropriate units. View Available Hint(s) M ? V2 = Value Units Submit Part A 986 ml NaCl solution is diluted to a volume of 1.29 L and a concentration of 6.00 M. What was the initial concentration C? Express your answer with the appropriate units. View Available Hint(s) w ? C = Value Units Submit Part A a 2.60 % (m/v) H2SO4 solution prepared from 11.2 mL of a 22.4 % H, SO4 solution. Express your answer to three significant figures and include the appropriate units. ? Value Units Submit Request Answer Part B a 0.45 M HCl solution prepared from 26 mL of a 7.3 M HCl solution Express your answer to two significant figures and include the appropriate units. HA ? Value Units Submit Request Answer Part C a 1.4 M NaOH solution prepared from 53.5 mL of a 17 M NaOH solution Express your answer to two significant figures and include the appropriate units. HA ? Value Units Submit Request Answer Part D a 1.5 % (m/v) CaCl, solution prepared from 22 mL of a 5.5 % (m/v) CaCl, solution Express your answer to two significant figures and include the appropriate units. H ? Value Units Submit Request Answer Part A 1.7 L of a 2.50 M HNO3 solution is added to water so that the final volume is 8.5 L. Express your answer to two significant figures and include the appropriate units. 0 ? Value Units Submit Request Answer Part B Water is added to 0.35 L of a 6.5 M NaF solution to make 2.4 L of a diluted NaF solution. Express your answer to two significant figures and include the appropriate units. HA ? Value Units Submit Request Answer Part C A 27.5 mL sample of an 9.0 % (m/v) KBr solution is diluted with water so that the final volume is 236.5 mL. Express your answer to two significant figures and include the appropriate units. ? Value Units Submit Request Answer Part D A 7.0 mL sample of a 54.5 % (m/v) acetic acid (HC,H,O2) solution is added to water to give a final volume of 41 mL Express your answer to two significant figures and include the appropriate units. ? Value Units Submit Request Answer What is the final volume V2 in milliliters when 0.941 L of a 40.3 % (m/v) solution is diluted to 22.8 % (m/v)? Express your answer with the appropriate units. View Available Hint(s) M ? V2 = Value Units Submit Part A 986 ml NaCl solution is diluted to a volume of 1.29 L and a concentration of 6.00 M. What was the initial concentration C? Express your answer with the appropriate units. View Available Hint(s) w ? C = Value Units Submit Part A a 2.60 % (m/v) H2SO4 solution prepared from 11.2 mL of a 22.4 % H, SO4 solution. Express your answer to three significant figures and include the appropriate units. ? Value Units Submit Request Answer Part B a 0.45 M HCl solution prepared from 26 mL of a 7.3 M HCl solution Express your answer to two significant figures and include the appropriate units. HA ? Value Units Submit Request Answer Part C a 1.4 M NaOH solution prepared from 53.5 mL of a 17 M NaOH solution Express your answer to two significant figures and include the appropriate units. HA ? Value Units Submit Request Answer Part D a 1.5 % (m/v) CaCl, solution prepared from 22 mL of a 5.5 % (m/v) CaCl, solution Express your answer to two significant figures and include the appropriate units. H ? Value Units Submit Request Answer Part A 1.7 L of a 2.50 M HNO3 solution is added to water so that the final volume is 8.5 L. Express your answer to two significant figures and include the appropriate units. 0 ? Value Units Submit Request Answer Part B Water is added to 0.35 L of a 6.5 M NaF solution to make 2.4 L of a diluted NaF solution. Express your answer to two significant figures and include the appropriate units. HA ? Value Units Submit Request Answer Part C A 27.5 mL sample of an 9.0 % (m/v) KBr solution is diluted with water so that the final volume is 236.5 mL. Express your answer to two significant figures and include the appropriate units. ? Value Units Submit Request Answer Part D A 7.0 mL sample of a 54.5 % (m/v) acetic acid (HC,H,O2) solution is added to water to give a final volume of 41 mL Express your answer to two significant figures and include the appropriate units. ? Value Units Submit Request
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


