Question: HELP ME WITH BOTH QUESTIONS PLEASE AND MAKE SURE YOU GET THE RIGHT ANSWER. IF YOU CANT ANSWER BOTH, PLEASE JUST LEAVE IT. I WILL
HELP ME WITH BOTH QUESTIONS PLEASE AND MAKE SURE YOU GET THE RIGHT ANSWER. IF YOU CANT ANSWER BOTH, PLEASE JUST LEAVE IT.
I WILL RATE IT. :) 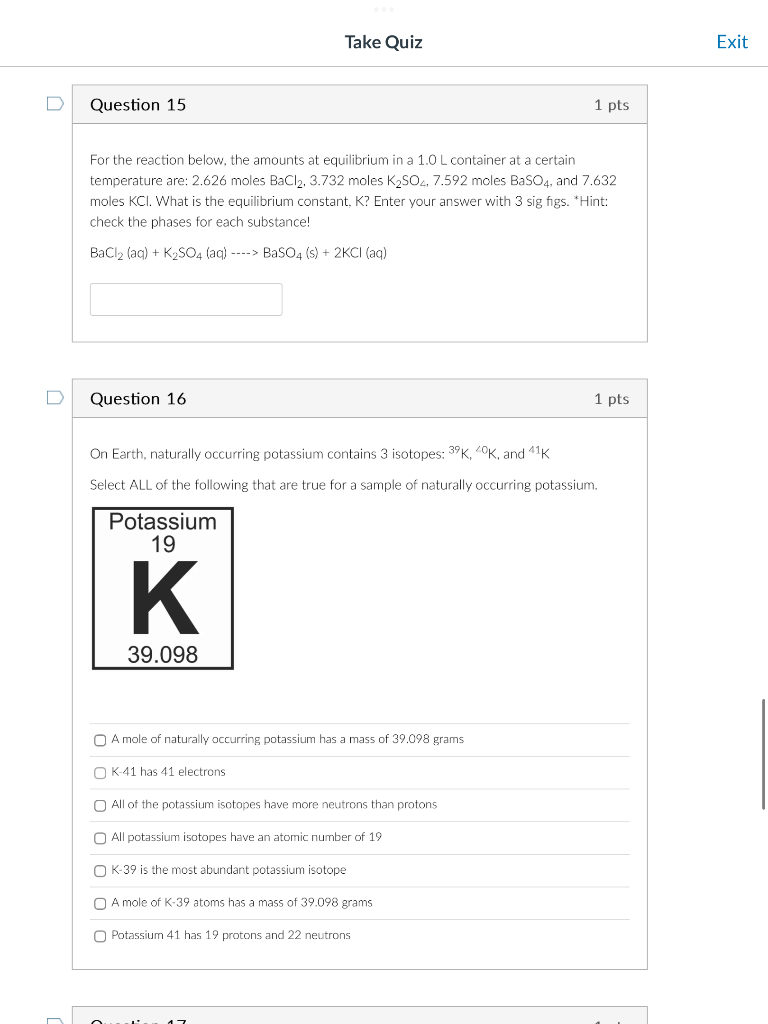
For the reaction below, the amounts at equilibrium in a 1.0L container at a certain temperature are: 2.626 moles BaCl2,3.732 moles K2SO4,7.592 moles BaSO4, and 7.632 moles KCl. What is the equilibrium constant, K? Enter your answer with 3 sig figs. Hint: check the phases for each substance! BaCl2(aq)+K2SO4(aq)>BaSO4(s)+2KCl(aq) Question 16 1 pts On Earth, naturally occurring potassium contains 3 isotopes: 39K,20K, and 41K Select ALL of the following that are true for a sample of naturally occurring potassium. A mole of naturaly occurring potassium has a mass of 39.098 grams K-41 has 41 electrons All of the potassium isotopes have more neutrons than protons All potassium isotopes have an atomic number of 19 K39 is the most abundant potassium isotope A mole of K-39 atoms has a mass of 39.098 grams Potassium 41 has 19 protons and 22 neutrons
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


