Question: HELP ME WITH BOTH QUESTIONS PLEASE AND MAKE SURE YOU GET THE RIGHT ANSWER. IF YOU CANT ANSWER BOTH, PLEASE JUST LEAVE IT. I WILL
HELP ME WITH BOTH QUESTIONS PLEASE AND MAKE SURE YOU GET THE RIGHT ANSWER. IF YOU CANT ANSWER BOTH, PLEASE JUST LEAVE IT.
I WILL RATE IT. :)
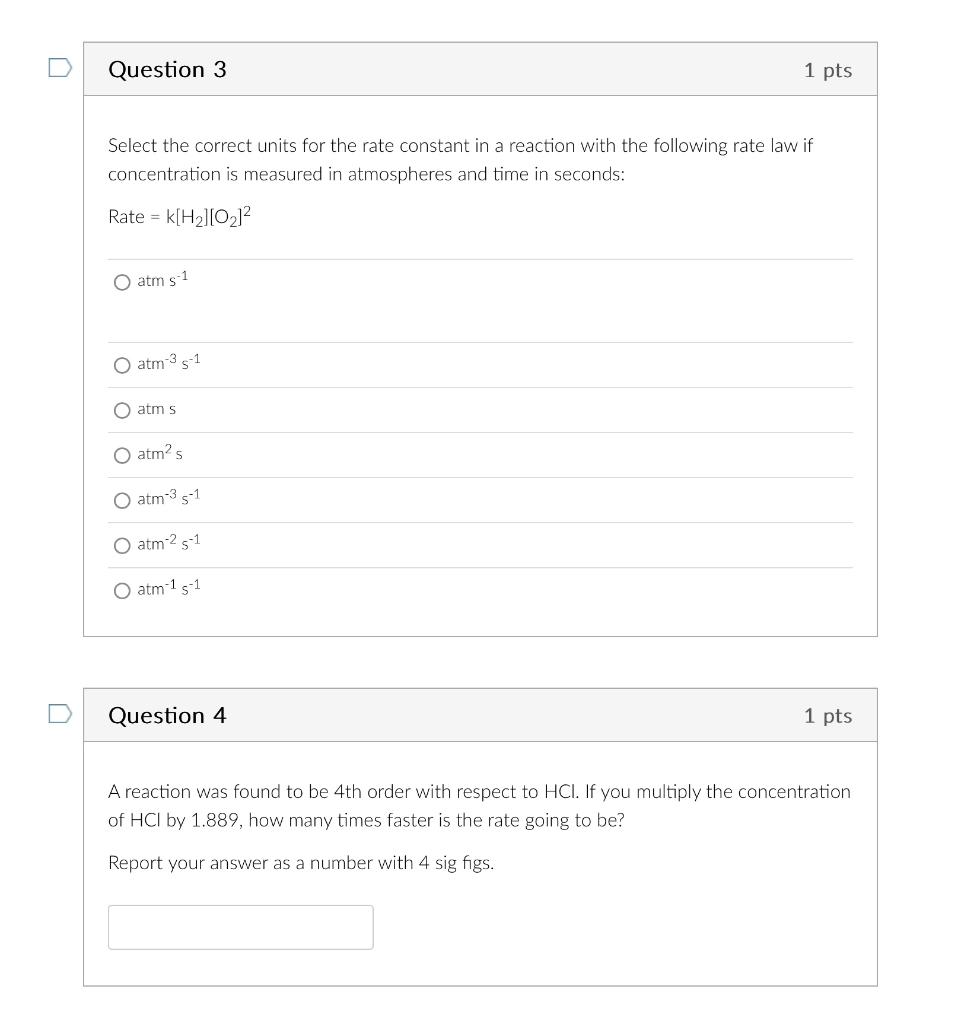
Select the correct units for the rate constant in a reaction with the following rate law if concentration is measured in atmospheres and time in seconds: Rate=k[H2][O2]2 atms1 atm3s1 atms atm2s atm3s1 atm2s1 atm1s1 Question 4 1 pts A reaction was found to be 4th order with respect to HCl. If you multiply the concentration of HCl by 1.889, how many times faster is the rate going to be? Report your answer as a number with 4 sig figs
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


