Question: help pls Use the References to access important values if needed for this question. The freezing point of water H2O is 0.00C at 1 atmosphere.
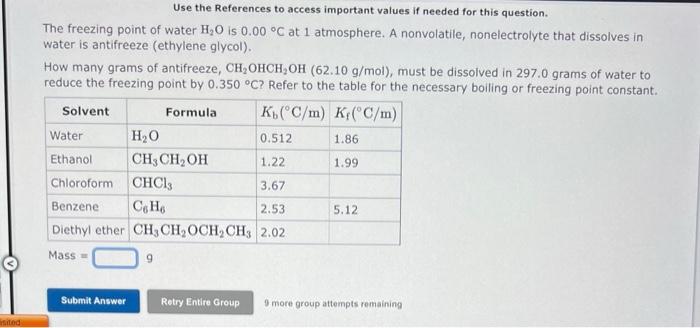
Use the References to access important values if needed for this question. The freezing point of water H2O is 0.00C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in water is antifreeze (ethylene glycol). How many grams of antifreeze, CH2OHCH2OH(62.10g/mol), must be dissolved in 297.0grams of water to reduce the freezing point by 0.350C ? Refer to the table for the necessary boiling or freezing point constant. Mass =9 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


