Question: Please help with these 2. Thanks. Use the References to access important values if needed for this question. The freezing point of benzene C6H6 is
Please help with these 2.
Thanks.
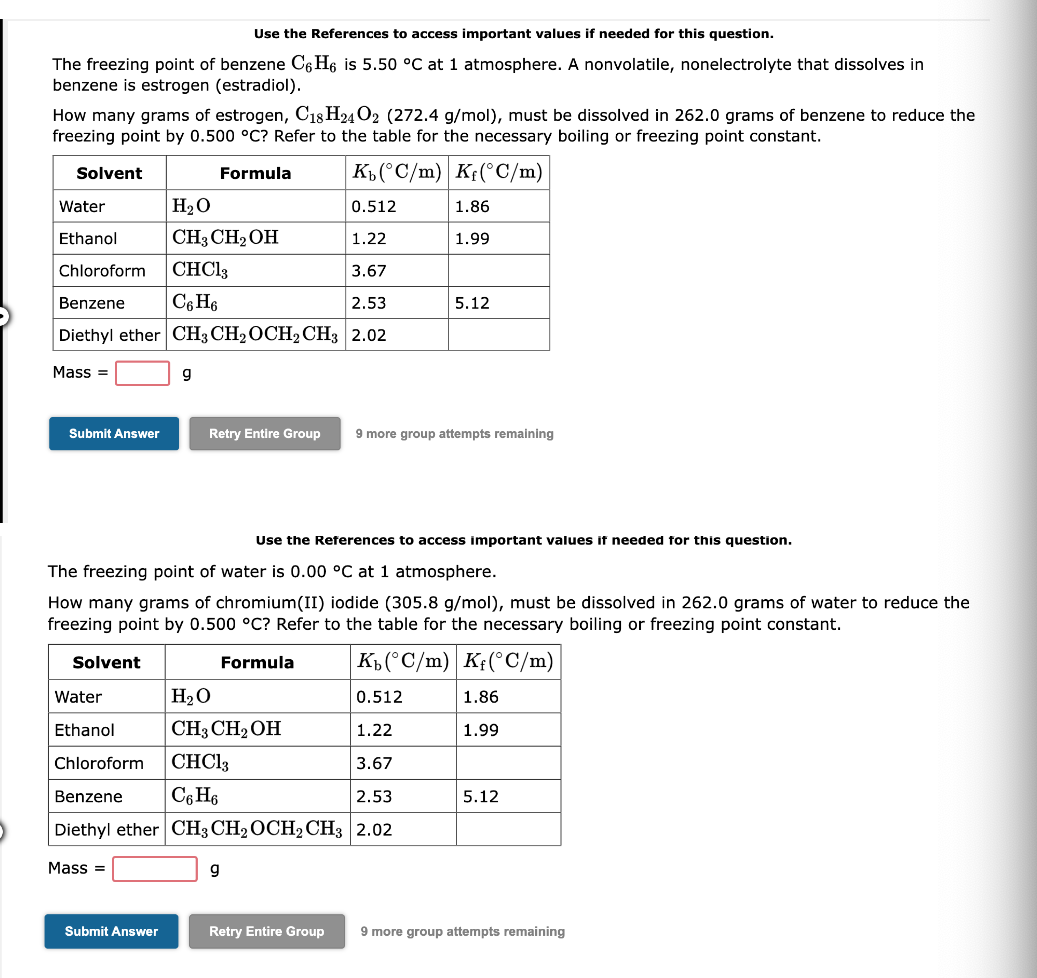
Use the References to access important values if needed for this question. The freezing point of benzene C6H6 is 5.50C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in benzene is estrogen (estradiol). How many grams of estrogen, C18H24O2(272.4g/mol), must be dissolved in 262.0grams of benzene to reduce the freezing point by 0.500C ? Refer to the table for the necessary boiling or freezing point constant. Mass =g 9 more group attempts remaining Use the References to access important values if needed for this question. The freezing point of water is 0.00C at 1 atmosphere. How many grams of chromium(II) iodide ( 305.8g/mol ), must be dissolved in 262.0 grams of water to reduce the freezing point by 0.500C ? Refer to the table for the necessary boiling or freezing point constant. Mass =g 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


