Question: Use the References to access important values if needed for this question. The freezing point of ethanol CH3CH2OH is 117.30C at 1 atmosphere. A nonvolatile,
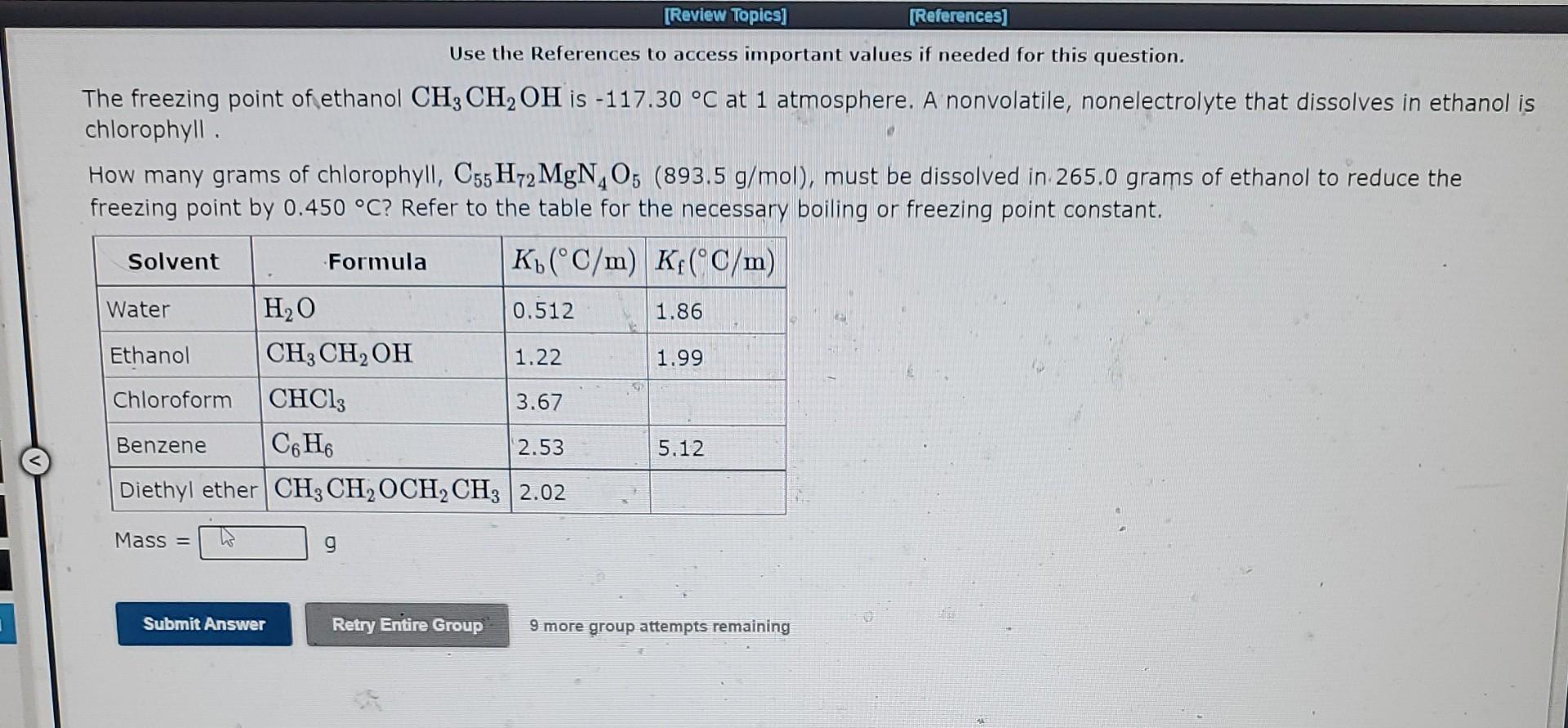
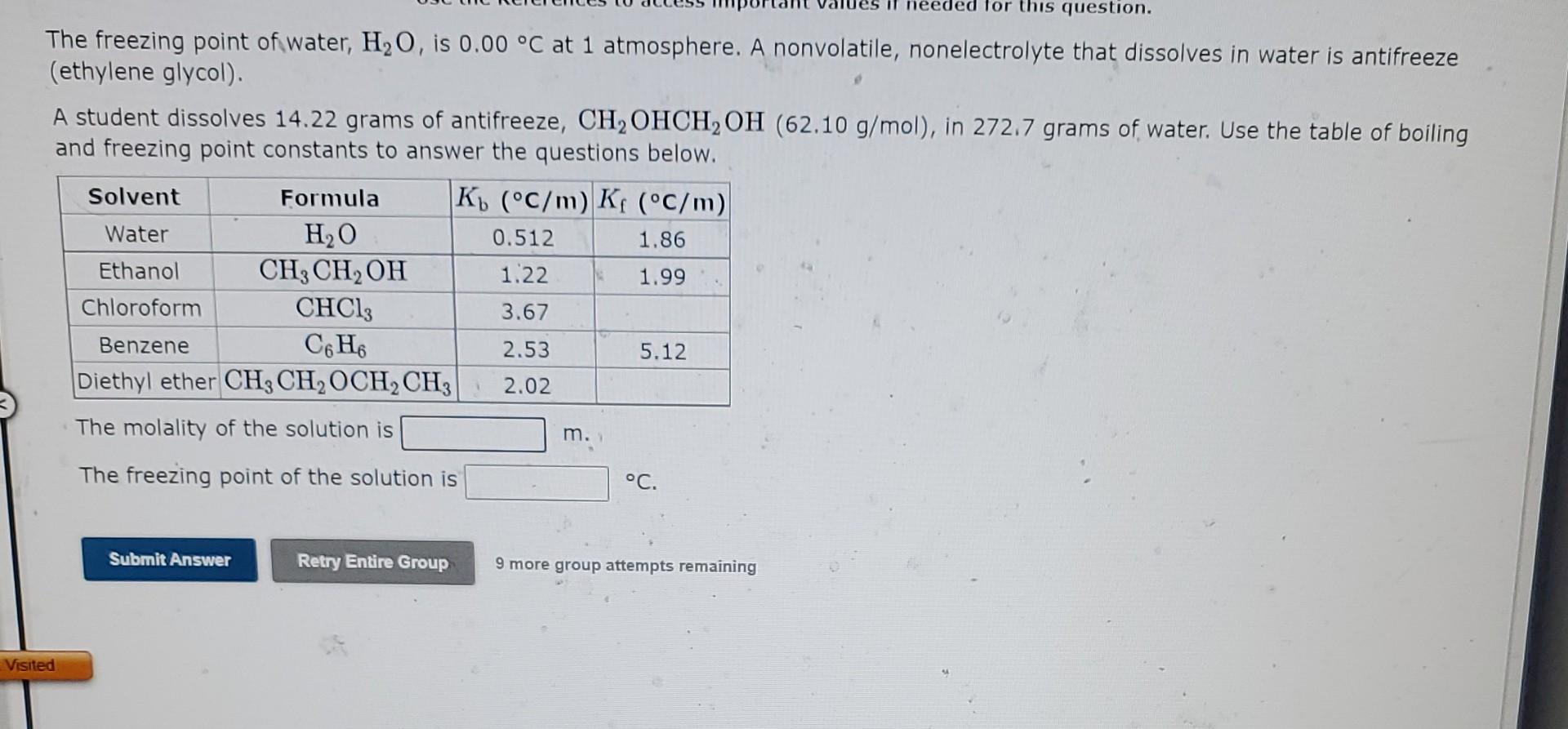
Use the References to access important values if needed for this question. The freezing point of ethanol CH3CH2OH is 117.30C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in ethanol chlorophyll. How many grams of chlorophyll, C55H72MgN4O5(893.5g/mol), must be dissolved in 265.0 grams of ethanol to reduce the freezing point by 0.450C ? Refer to the table for the necessary boiling or freezing point constant. The freezing point of water, H2O, is 0.00C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in water is antifreeze (ethylene glycol). A student dissolves 14.22 grams of antifreeze, CH2OHCH2OH(62.10g/mol), in 272.7 grams of water. Use the table of boiling and freezing point constants to answer the questions below. The molality of the solution is The freezing point of the solution is C. 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


