Question: help thermo, with steps please! 1. The following plot shows values of excess Gibbs energy over the ideal gas constant, 85/R in [K], for mixtures
help thermo, with steps please!
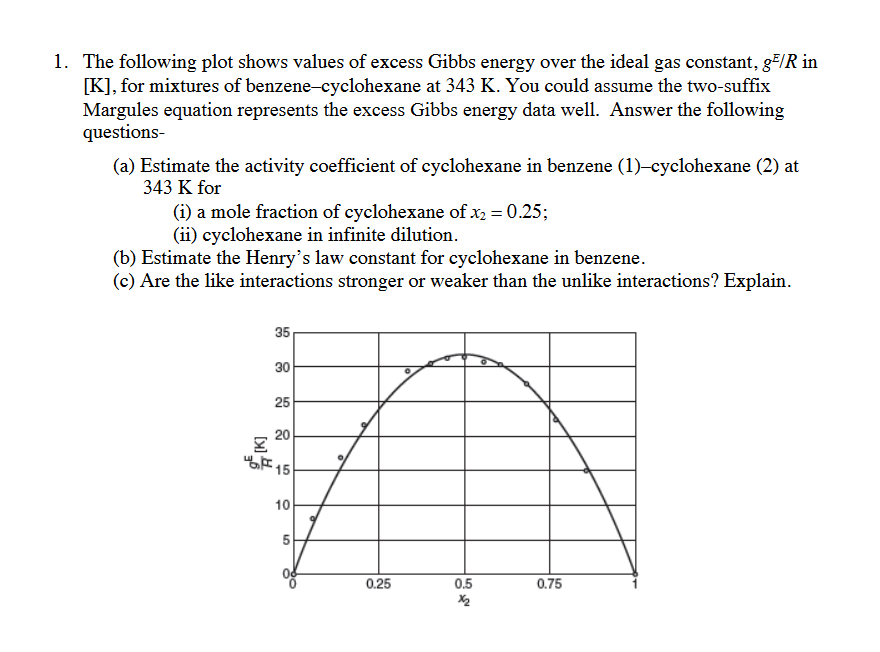
1. The following plot shows values of excess Gibbs energy over the ideal gas constant, 85/R in [K], for mixtures of benzene-cyclohexane at 343 K. You could assume the two-suffix Margules equation represents the excess Gibbs energy data well. Answer the following questions- (a) Estimate the activity coefficient of cyclohexane in benzene (1)-cyclohexane (2) at 343 K for (i) a mole fraction of cyclohexane of x2 = 0.25; (ii) cyclohexane in infinite dilution. (b) Estimate the Henry's law constant for cyclohexane in benzene. (c) Are the like interactions stronger or weaker than the unlike interactions? Explain. = 35 30 25 20 I b 15 10 5 Oo 0.25 0.75 0.5 X2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


