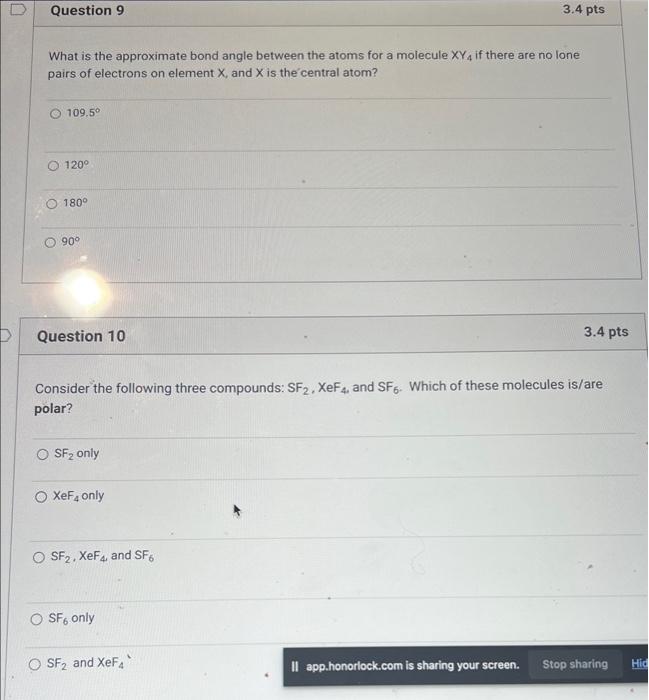
Question: help with 9&10 What is the approximate bond angle between the atoms for a molecule XY4 if there are no lone pairs of electrons on
 help with 9&10
help with 9&10What is the approximate bond angle between the atoms for a molecule XY4 if there are no lone pairs of electrons on element X, and X is the central atom? 109.50 120 180 90 Question 10 3.4pts Consider the following three compounds: SF2,XeF4 and SF6. Which of these molecules is/are polar? SF2 only XeF4 only SF2,XeF4, and SF6 SF6 only SF2 and XeF4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


