Question: Help with solubility! a) write a chemical equation that relates calcite solubility (as represented by Ca2+) to the partial pressure of CO2 b) calculate the
Help with solubility!
a) write a chemical equation that relates calcite solubility (as represented by Ca2+) to the partial pressure of CO2


b) calculate the concentration of dissolved calcium in equilibrium with calcite at 25C for the following log10 partial pressures of CO2. Assume activity (1st is pre industry atmosphere, 2nd current atmosphere, 3rd soil atmoshphere) = concentration for these calculations
| log pCO2 |
| -3.57 1st |
| -3.39 2nd |
| -1.51 3rd |
| Ca2+, mmol/L, in equilibrium with calcite |
| ? |
| ? |
| ? |
c) what do these calculations mean about the CO2-calcite equilibrium-Ca concentration in the various atmospheres?
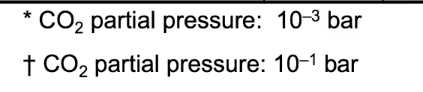
Mineral Dissociation reaction log (Keg) Solubility @ pH 7 mg L-1 Calcite CaCO3 Ca2+ + + CO32- -8.4 100*; 500+ * CO2 partial pressure: 10-3 bar CO2 partial pressure: 10-1 bar Mineral Dissociation reaction log (Keg) Solubility @ pH 7 mg L-1 Calcite CaCO3 Ca2+ + + CO32- -8.4 100*; 500+ * CO2 partial pressure: 10-3 bar CO2 partial pressure: 10-1 bar
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


