Question: Hess's Law: Few notes A state function is a function that depends only on initial and final conditions and not the number of steps required
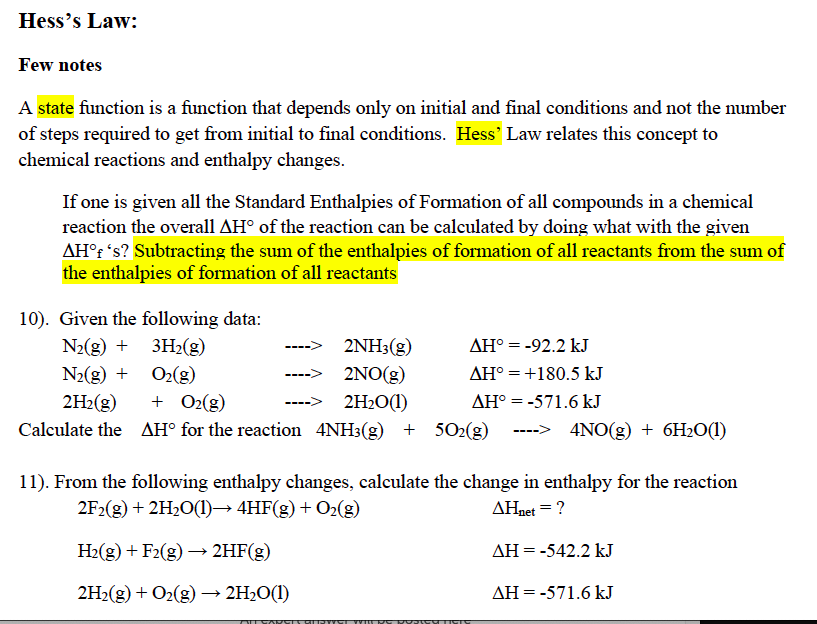
Hess's Law: Few notes A state function is a function that depends only on initial and final conditions and not the number of steps required to get from initial to final conditions. Hess' Law relates this concept to chemical reactions and enthalpy changes. If one is given all the Standard Enthalpies of Formation of all compounds in a chemical reaction the overall AH of the reaction can be calculated by doing what with the given AHf 's? Subtracting the sum of the enthalpies of formation of all reactants from the sum of the enthalpies of formation of all reactants 10). Given the following data: N2(g) + 3H2(g) 2NH3(g) AH = -92.2 kJ N2(g) + 02(9) ----> 2NO(g) AH = +180.5 kJ 2H2(g) + O2(g) 2H2O(1) AH = -571.6 kJ Calculate the AH for the reaction 4NH3(g) + 502(g) ----> 4NO(g) + 6H2O(1) 11). From the following enthalpy changes, calculate the change in enthalpy for the reaction 2F2(g) + 2H2O(1) 4HF(g) + O2(g) AHnet = ? H2(g) + F2(g) 2HF(g) AH = -542.2 kJ 2H2(g) + O2(g) 2H2O(1) AH=-571.6 kJ =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


