Question: Hey, I need help with the following question from pharmaceutical analytical chemistry! Thank you! You will use reverse phase liquid chromatography (RP- HPLC) to perform

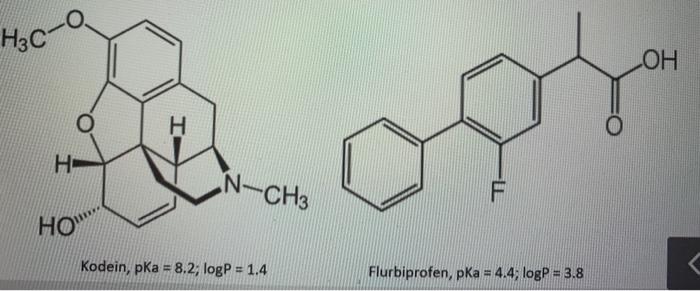
Hey, I need help with the following question from pharmaceutical analytical chemistry! Thank you! You will use reverse phase liquid chromatography (RP- HPLC) to perform a separation of a sample containing codeine and flurbiprofen (see structure below). As a stationary phase you use a C18 column, and as a mobile phase a 10:90 mixture of methanol: 0.1 M phosphate buffer with pH 2 and total ionic strength 0.1 M. a. Reason yourself, based on the structure and properties of the analytes and the experimental system, to the order in which the two analytes should elute (ie leave the column and be detected) when you perform the separation. Please justify your answer! b. explain how the retention of the two analytes will change if you raise the pH of the buffer to pH 11 (which results in the mobile phase composition becoming a 10:90 mixture of methanol 0.1 M phosphate buffer with pH13 and the total ionic strength 0.1 M). Remember to justify your answer! H3C . N-CH HO"" Kodein, pKa = 8.2, logP = 1.4 Flurbiprofen, pka = 4.4, logP = 3.8
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


