Question: HG is a hypothetical weak acid. Given that the pH at the equivalence point for the titration between 0.073 M potassium hydroxide and 20.0 mL
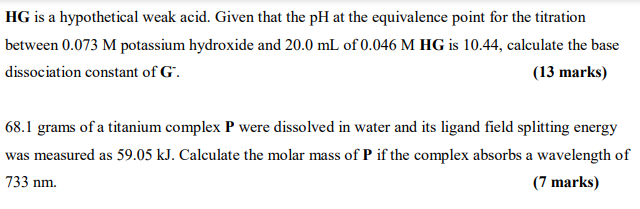
HG is a hypothetical weak acid. Given that the pH at the equivalence point for the titration between 0.073 M potassium hydroxide and 20.0 mL of 0.046 M HG is 10.44, calculate the base dissociation constant of G. (13 marks) 68.1 grams of a titanium complex P were dissolved in water and its ligand field splitting energy was measured as 59.05 kJ. Calculate the molar mass of P if the complex absorbs a wavelength of 733 nm. (7 marks)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


