Question: hi can you help me for this exercice pls Exercise 1: Ionic or covalent? When combining the following pairs of elements, which type of compound
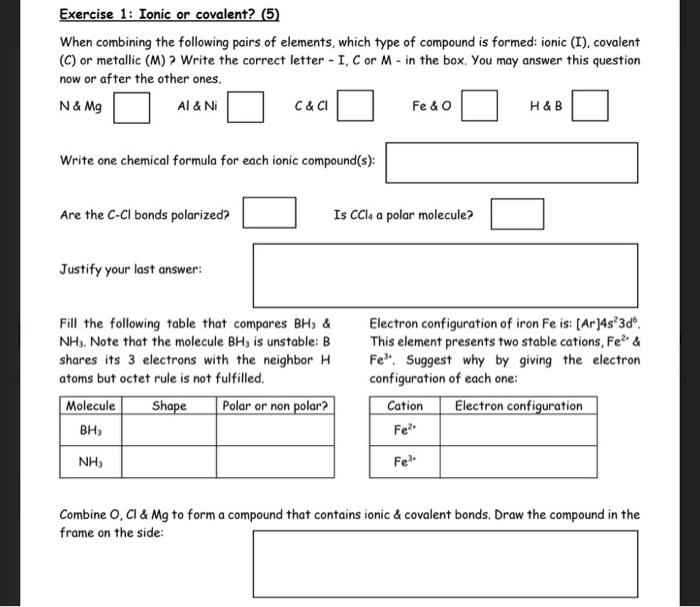
Exercise 1: Ionic or covalent? When combining the following pairs of elements, which type of compound is formed: ionic (I), covalent (C) or metallic (M)? Write the correct letter - I, C or M - in the box. You may answer this question now or after the other ones. N&Mg Al&Ni C&Cl Fe&O H&B Write one chemical formula for each ionic compound(s): Are the CCl bonds polarized? Is CCl4 a polar molecule? Justify your last answer: Fill the following table that compares BH3& Electron configuration of iron Fe is: [Ar2]4s23d6. NH3. Note that the molecule BH3 is unstable: B This element presents two stable cations, Fe2+ \& shares its 3 electrons with the neighbor HFe3. Suggest why by giving the electron atoms but octet rule is not fulfilled. configuration of each one: Combine O, Cl&Mg to form a compound that contains ionic \& covalent bonds. Draw the compound in the frame on the side
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


