Question: hi expert please answer this question asap needed urgently will do thumbs up immediately .thank you On a 5 day wilderness expedition you will need
 hi expert please answer this question asap needed urgently will do thumbs up immediately .thank you
hi expert please answer this question asap needed urgently will do thumbs up immediately .thank you
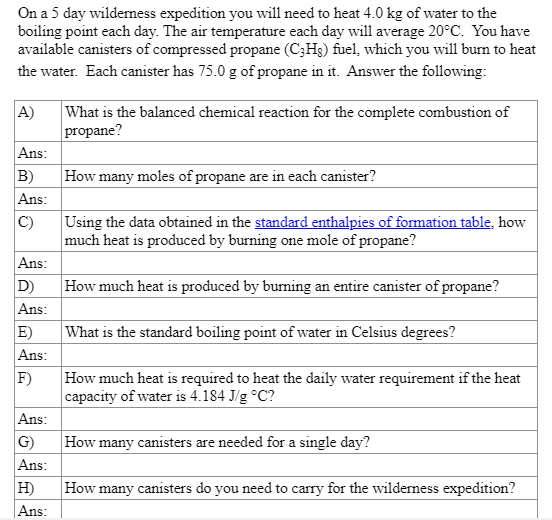
On a 5 day wilderness expedition you will need to heat 4.0 kg of water to the boiling point each day. The air temperature each day will average 20C. You have available canisters of compressed propane (CzHg) fuel, which you will burn to heat the water. Each canister has 75.0 g of propane in it. Answer the following: A) What is the balanced chemical reaction for the complete combustion of propane? How many moles of propane are in each canister? Ans: B) Ans: C) Using the data obtained in the standard enthalpies of formation table, how much heat is produced by burning one mole of propane? Ans: D How much heat is produced by burning an entire canister of propane? What is the standard boiling point of water in Celsius degrees? Ans: E) Ans: F) How much heat is required to heat the daily water requirement if the heat capacity of water is 4.184 J/g C? Ans: How many canisters are needed for a single day? Ans: H) Ans: How many canisters do you need to carry for the wilderness expedition
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


