Question: Hi, I need help with this problem. Will appreciate a written solution. Thank you. A groundwater contains the following constituents: CO2=10mg/LCa2+=98mg/LMg2+=24mg/LHCO3=240mg/L The facility is to
Hi, I need help with this problem. Will appreciate a written solution. Thank you.
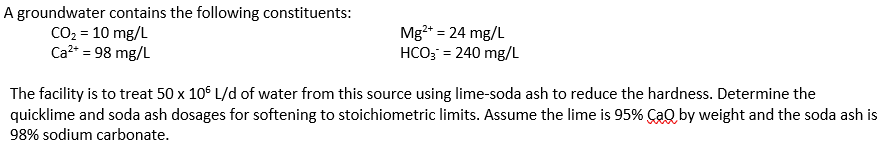
A groundwater contains the following constituents: CO2=10mg/LCa2+=98mg/LMg2+=24mg/LHCO3=240mg/L The facility is to treat 50106L/d of water from this source using lime-soda ash to reduce the hardness. Determine the quicklime and soda ash dosages for softening to stoichiometric limits. Assume the lime is 95% CaQ by weight and the soda ash is 98% sodium carbonate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


