Question: Hi, need help with a textbook homework question from my reaction process engineering course in chemical engineering. (Picture attached below) (a) Estimate the volume of
Hi, need help with a textbook homework question from my reaction process engineering course in chemical engineering. (Picture attached below)
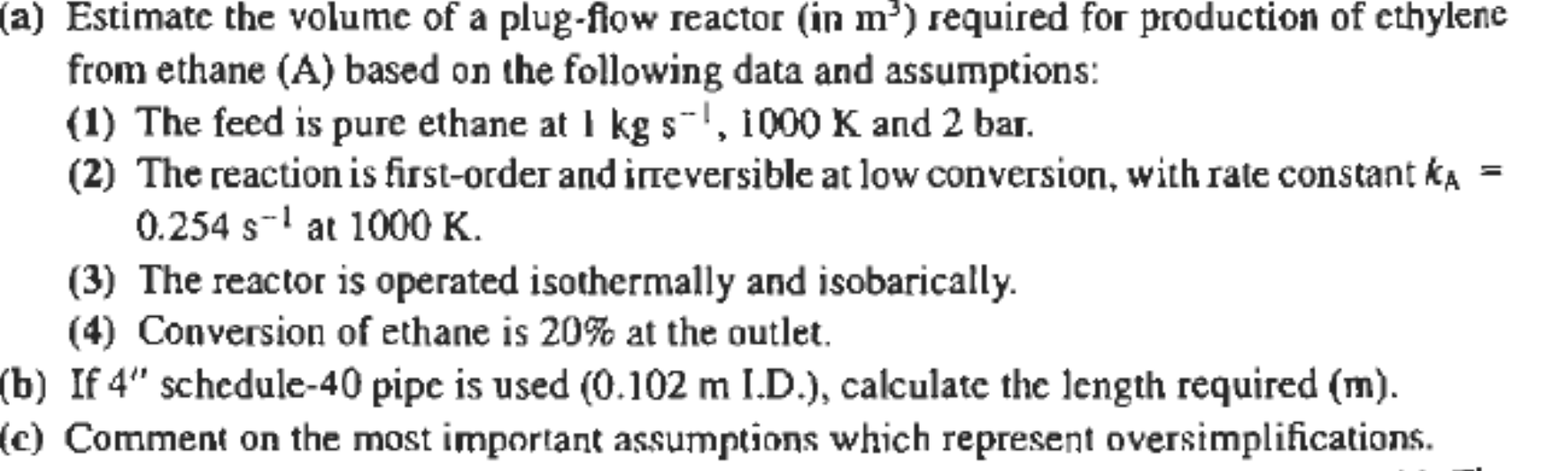
(a) Estimate the volume of a plug-fiow reactor (in m) required for production of ethylene from ethane (A) based on the following data and assumptions: (1) The feed is pure ethane at I kg s-!, 1000 K and 2 bar. (2) The reaction is first-order and irreversible at low conversion, with rate constant ka = 0.254 s-' at 1000 K. (3) The reactor is operated isothermally and isobarically. (4) Conversion of ethane is 20% at the outlet. (b) If 4" schedule-40 pipe is used (0.102 m I.D.), calculate the length required (m). (c) Comment on the most important assumptions which represent oversimplifications
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


