Question: Hi, need help with a textbook homework question from my reaction process engineering course in chemical engineering. (Picture attached below) 15-2 A first-order liquid-phase reaction
Hi, need help with a textbook homework question from my reaction process engineering course in chemical engineering. (Picture attached below)
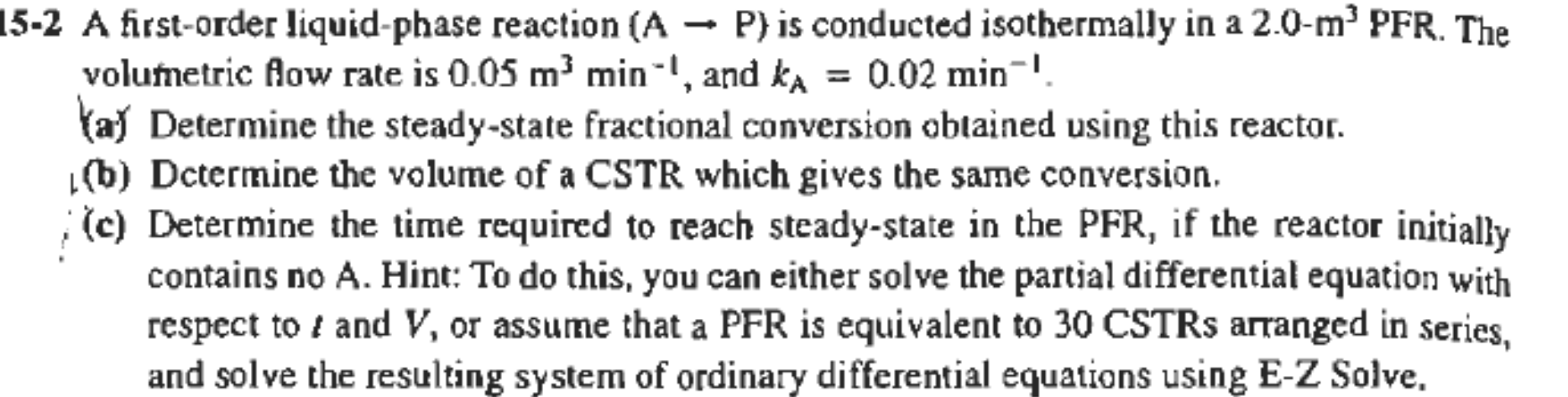
15-2 A first-order liquid-phase reaction (A P) is conducted isothermally in a 2.0-m' PFR. The volumetric Row rate is 0.05 m2 min, and ka = 0.02 min! Vay Determine the steady-state fractional conversion obtained using this reactor. (6) Dctermine the volume of a CSTR which gives the same conversion. (c) Determine the time required to reach steady-state in the PFR, if the reactor initially contains no A. Hint: To do this, you can either solve the partial differential equation with respect to i and V, or assume that a PFR is equivalent to 30 CSTRs arranged in series, and solve the resulting system of ordinary differential equations using E-Z Solve
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


