Question: Hi, please help with what you can, this is a practice for our final! h=6.626 x 10-34 J S c=2.998 x 108 m/s 1 amu
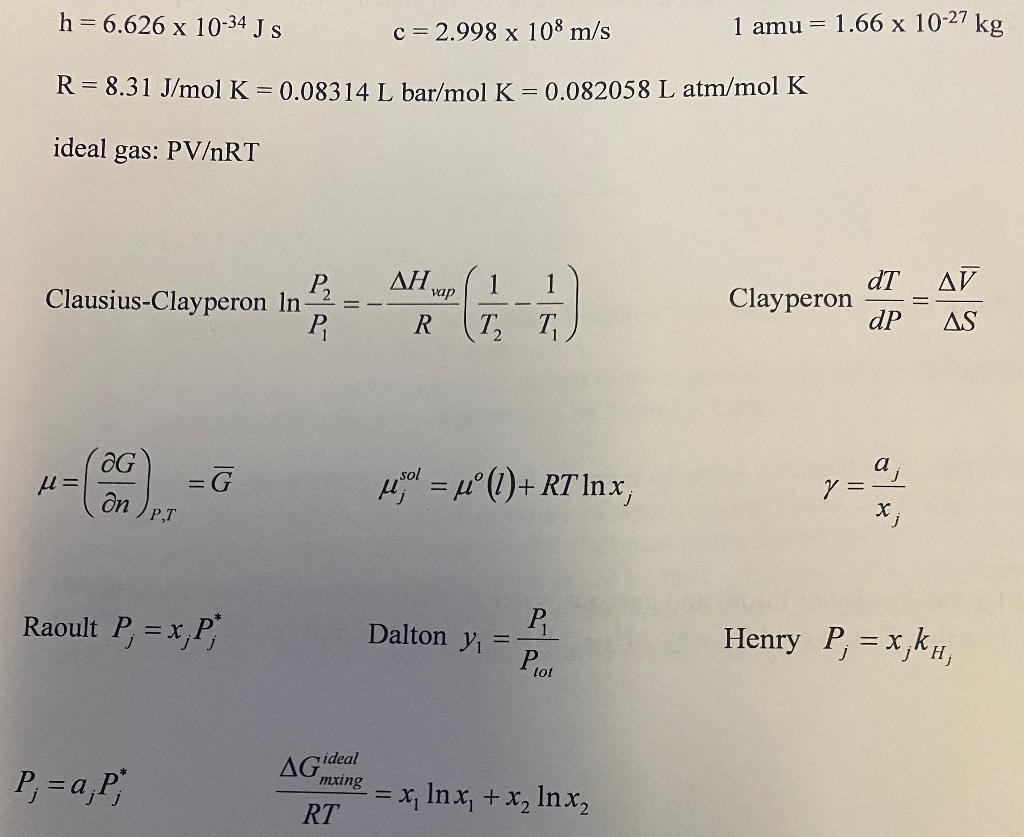
 Hi, please help with what you can, this is a practice for our final!
Hi, please help with what you can, this is a practice for our final!
h=6.626 x 10-34 J S c=2.998 x 108 m/s 1 amu = 1.66 x 10-27 kg R= 8.31 J/mol K = 0.08314 L bar/mol K = 0.082058 L atm/mol K ideal gas: PVRT al vap dT AV Clausius-Clayperon In? 1 1 T T Clayperon P, R dP AS G u= =G Y = 1 ()+ R7 Inx an a y = X; PT Raoult P, = x,P Dalton y P = Henry P = x,kh, Piot AG ideal P;= a;P, mxing = x, In x, + x, Inxz RT a 5. (15 points) Benzene and toluene form an ideal solution for all concentrations at 25 C. At this temperature, pure benzene has a vapor pressure of 190 torr and pure toluene has a vapor pressure of 60 torr. Using a mole fraction of 0.25 for benzene, determine the composition of the vapor phase and total pressure of the solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


