Question: Hi, please help with what you can, this is a practice for our final! h=6.626 x 10-34 J S c=2.998 x 108 m/s 1 amu
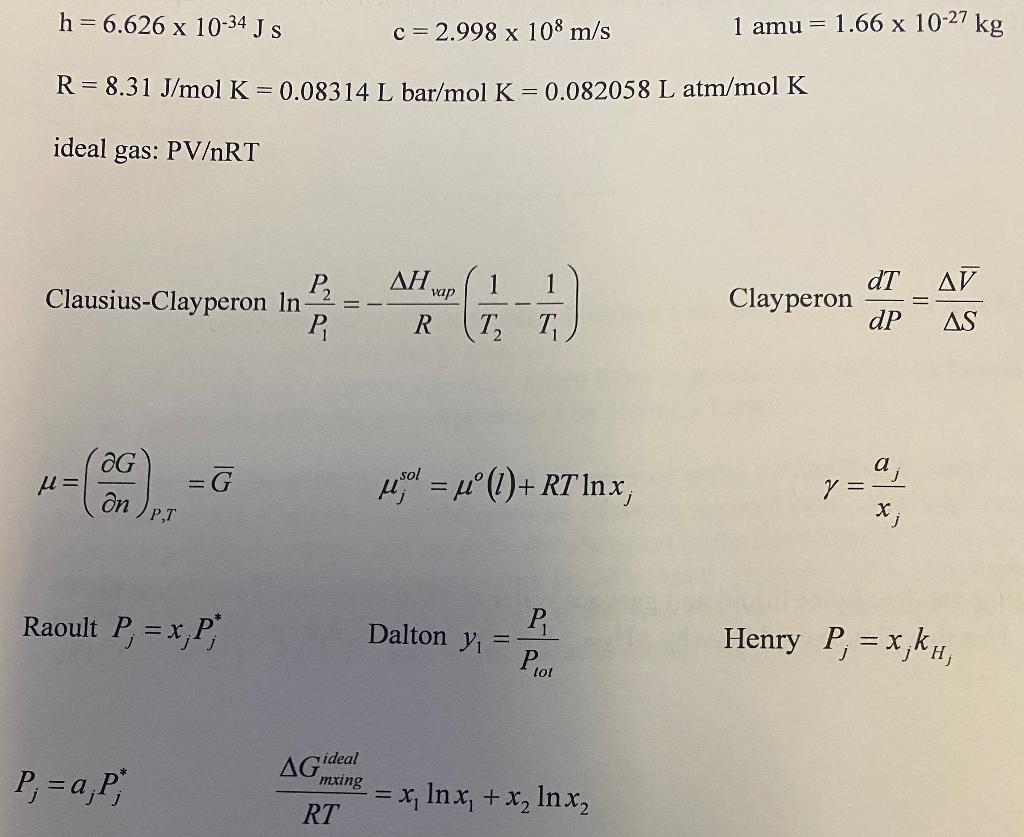
Hi, please help with what you can, this is a practice for our final!
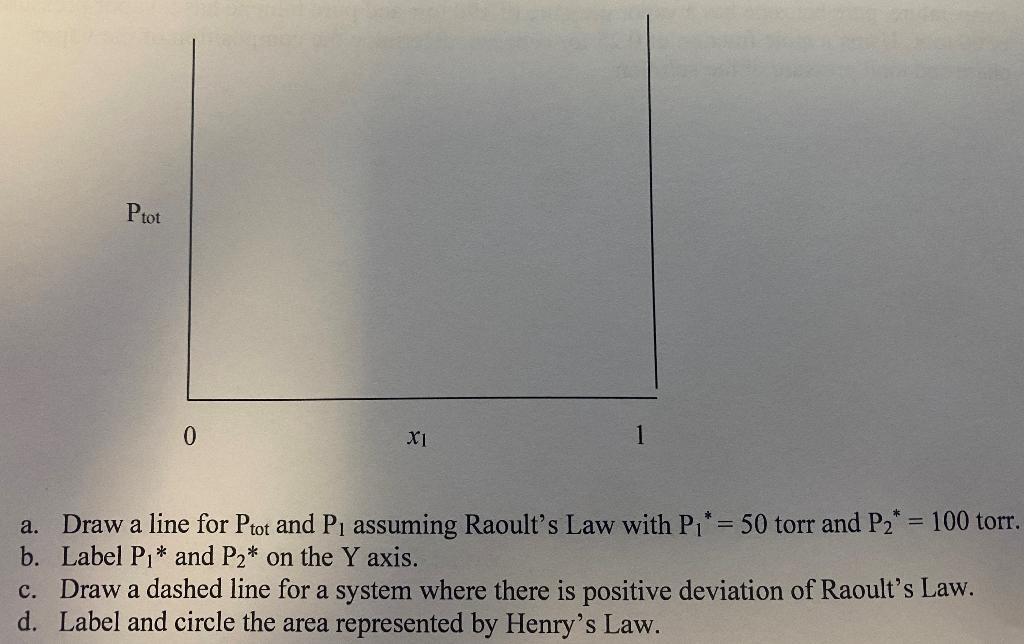
h=6.626 x 10-34 J S c=2.998 x 108 m/s 1 amu = 1.66 x 10-27 kg R= 8.31 J/mol K = 0.08314 L bar/mol K = 0.082058 L atm/mol K ideal gas: PVRT al vap dT AV Clausius-Clayperon In? 1 1 T T Clayperon P, R dP AS G u= =G Y = 1 ()+ R7 Inx an a y = X; PT Raoult P, = x,P Dalton y P = Henry P = x,kh, Piot AG ideal P;= a;P, mxing = x, In x, + x, Inxz RT Ptot 0 X1 1 a a. Draw a line for Ptot and Pi assuming Raoult's Law with P1* = 50 torr and P2 * = 100 torr. b. Label P,* and P2* on the Y axis. c. Draw a dashed line for a system where there is positive deviation of Raoult's Law. d. Label and circle the area represented by Henry's Law
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


