Question: Hi, please help with what you can, this is a practice for our final! h=6.626 x 10-34 J S c=2.998 x 108 m/s 1 amu
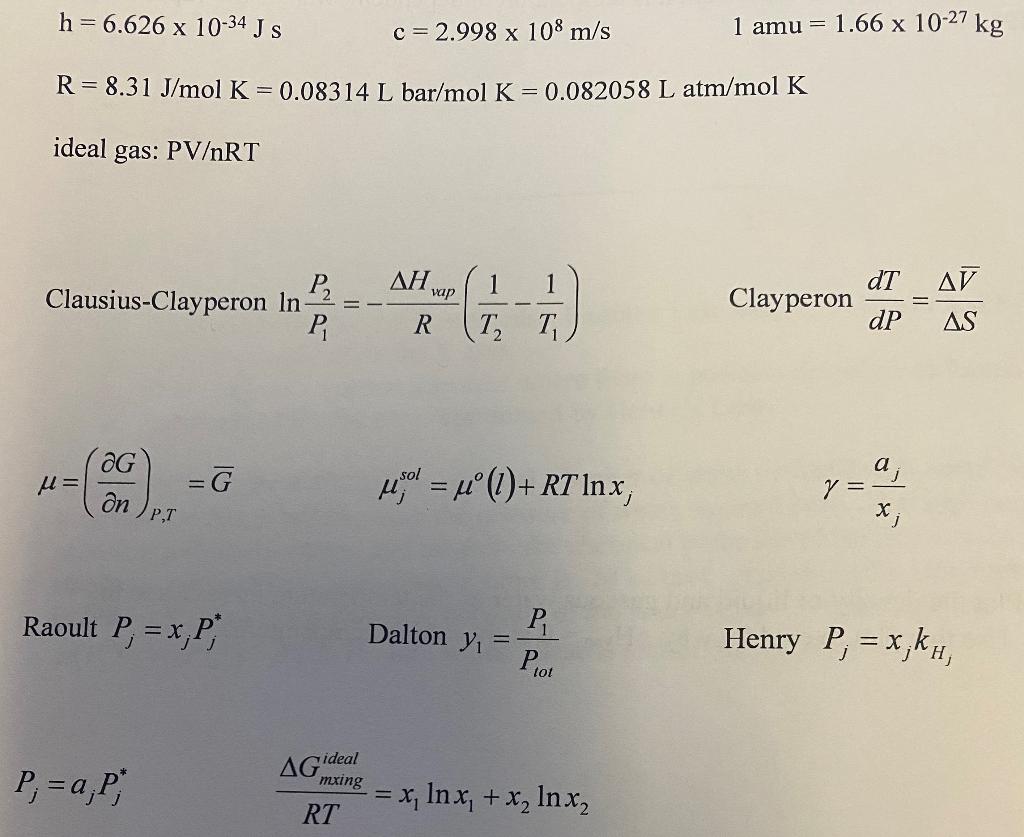

Hi, please help with what you can, this is a practice for our final!
h=6.626 x 10-34 J S c=2.998 x 108 m/s 1 amu = 1.66 x 10-27 kg R= 8.31 J/mol K = 0.08314 L bar/mol K = 0.082058 L atm/mol K ideal gas: PVRT al vap dT AV Clausius-Clayperon In? 1 1 T T Clayperon P, R dP AS G u= =G Y = 1 ()+ R7 Inx an a y = X; PT Raoult P, = x,P Dalton y P = Henry P = x,kh, Piot AG ideal P;= a;P, mxing = x, In x, + x, Inxz RT 9. (15 points) Derive the Maxwell relation that occurs from G(P,T) given that dG =-SDT +VAP a. Write the formal derivative of G(P,T) below. b. Using the differential form of Gibbs energy above, make two relationships using these two equations. c. Find the Maxwell relationship
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


