Question: Hi , Pls help me to solve the solution step by step with calculation method details and latest syllabus This is Thermodynamics subject.. Thanks 7.(a)
Hi , Pls help me to solve the solution step by step with calculation method details and latest syllabus
This is Thermodynamics subject.. Thanks

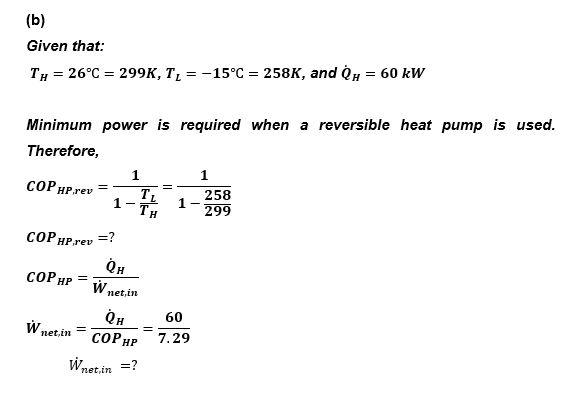
7.(a) P1V1=P2V2P2=(V2V1)P1P2=(21)1.5=? This is a closed system while the process which takes place is a non-cyclic process. Hence, the energy balance equation applied here is: Qnet,inWnet,tut=U U=mu=mcv(T2T1) according to the specific heat relation for ideal gas (Refer to Section 2.5), Hence QnetinWnet,out=mcv(T2T1)=0Qnet,in=WnetoutQnetin=Wail,outWinQnetin=mRT1ln(P1P2)Win=(0.8)(287)(303)ln(1.50.75)45103=? Given that: TH=26C=299K,TL=15C=258K,andQH=60kW Minimum power is required when a reversible heat pump is used. Therefore, COPHP,rev=1THTL1=12992581COPHP,rev=?COPHP=Wnet,inQHWnet,in=COPHPQH=7.2960Wnet,in=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


