Question: Homework #1: Ch24 Spring 2021/2022 Estimate the gas phase diffusion coefficient of ammonia (NH3) in Methanol at 1.0 atm and 373K by making use of
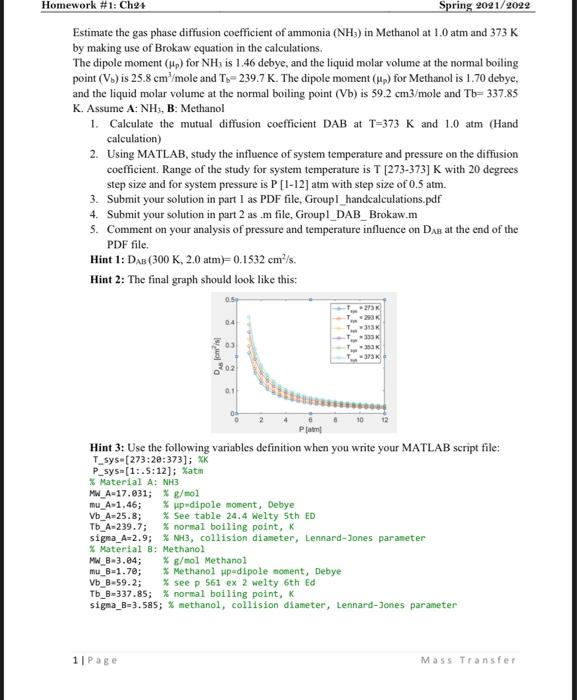
Homework #1: Ch24 Spring 2021/2022 Estimate the gas phase diffusion coefficient of ammonia (NH3) in Methanol at 1.0 atm and 373K by making use of Brokaw equation in the calculations. The dipole moment (Hp) for NH3 is 1.46 debye, and the liquid molar volume at the normal boiling point (V.) is 25.8 cm /mole and T-239.7 K. The dipole moment () for Methanol is 1.70 debye. and the liquid molar volume at the normal boiling point (Vb) is 59.2 cm3/mole and Th=337.85 K. Assume A:NH, B: Methanol 1. Calculate the mutual diffusion coefficient DAB at T=373 K and 1.0 atm (Hand calculation) 2. Using MATLAB, study the influence of system temperature and pressure on the diffusion coefficient. Range of the study for system temperature is T [273-373] K with 20 degrees step size and for system pressure is P [1-12) atm with step size of 0.5 atm. 3. Submit your solution in part 1 as PDF file, GroupI_handcalculations.pdf 4. Submit your solution in part 2 as .m file, Groupl_DAB_Brokaw.m 5. Comment on your analysis of pressure and temperature influence on Dag at the end of the PDF file. Hint 1: DAB (300 K, 2.0 atm=0.1532 cmls. Hint 2: The final graph should look like this: 05 -273 293 313 333K 33% - 373 03 son's 01 0 2 P Hint 3: Use the following variables definition when you write your MATLAB script file: 1_sys-[273:20:373); XK P_sys=(1:.5:12); matm % Material A: NH3 MW_A-17.031; * g/mol mu_A 1.46; % up-dipole moment, Debye Vb_A-25.8; % See table 24.4 Welty 5th ED Tb_A-239.7; % normal boiling point, K sigma_A-2.9; % NH3, collision diameter, Lennard-Jones parameter % Material B: Methanol MW_8-3.84; * g/mol Methanol mu_3-1.70; * Methanol wpadipole moment, Debye Vb_8-59.2; % see p 561 ex 2 welty 6th Ed Tb_B-337.85; % normal boiling point, K signa_B=3.585; * methanol, collision diameter, Lennard-Jones parameter 1 Page Mass Transfer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


