Question: can u solve mass transfere Estimate the gas phase diffusion coefficient of ammonia (NH3) in Methanol at 1.0 atm and 373 K by making use
can u solve mass transfere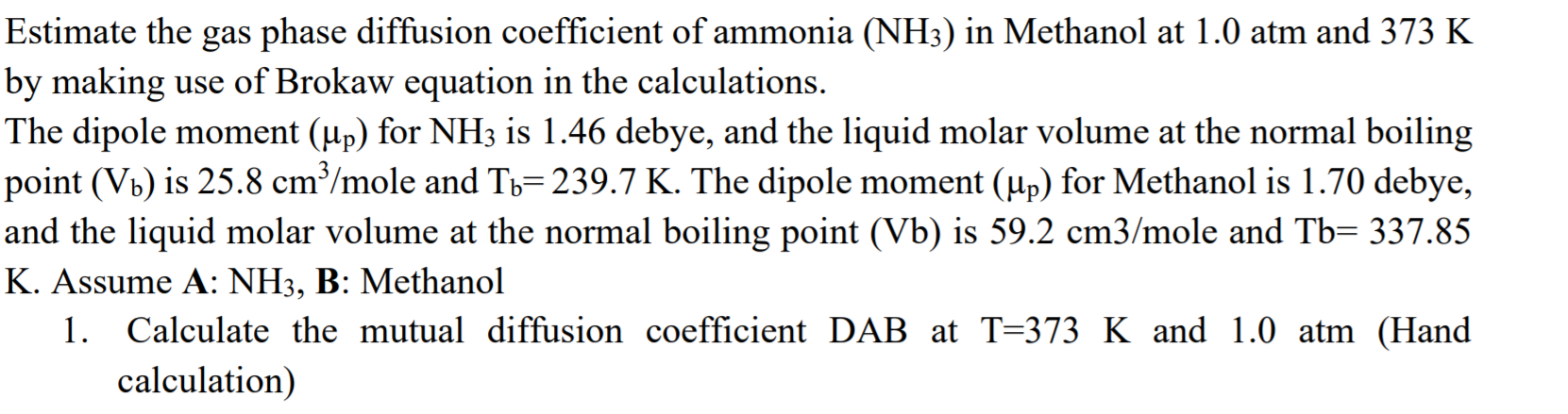
Estimate the gas phase diffusion coefficient of ammonia (NH3) in Methanol at 1.0 atm and 373 K by making use of Brokaw equation in the calculations. The dipole moment (up) for NH3 is 1.46 debye, and the liquid molar volume at the normal boiling point (Vb) is 25.8 cmy/mole and Tb=239.7 K. The dipole moment (up) for Methanol is 1.70 debye, and the liquid molar volume at the normal boiling point (Vb) is 59.2 cm3/mole and Tb= 337.85 K. Assume A: NH3, B: Methanol 1. Calculate the mutual diffusion coefficient DAB at T=373 K and 1.0 atm (Hand calculation)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


