Question: How can we define triple point in the following phase diagram Triple Point the triple point, which is the temperature and pressure where three phases
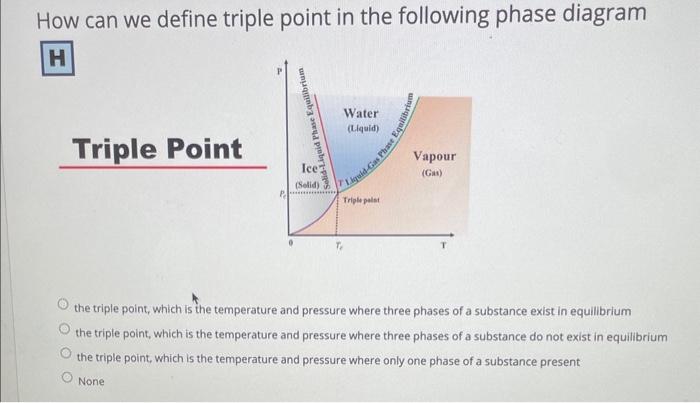
How can we define triple point in the following phase diagram Triple Point the triple point, which is the temperature and pressure where three phases of a substance exist in equilibrium the triple point, which is the temperature and pressure where three phases of a substance do not exist in equilibrium the triple point, which is the temperature and pressure where only one phase of a substance present
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


