Question: How can we define triple point in the following phase diagram the triple point, which is the temperature and pressure where three phases of a
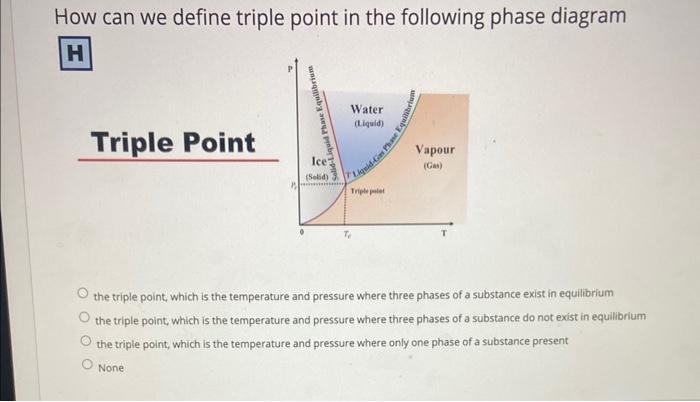
How can we define triple point in the following phase diagram the triple point, which is the temperature and pressure where three phases of a substance exist in equilibrium the triple point, which is the temperature and pressure where three phases of a substance do not exist in equilibrium the triple point, which is the temperature and pressure where only one phase of a substance present. None
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


