Question: how do i answer the last question? (its the last picture) please show a step by step explaination. thankyou In chemical reactions, heat is converted

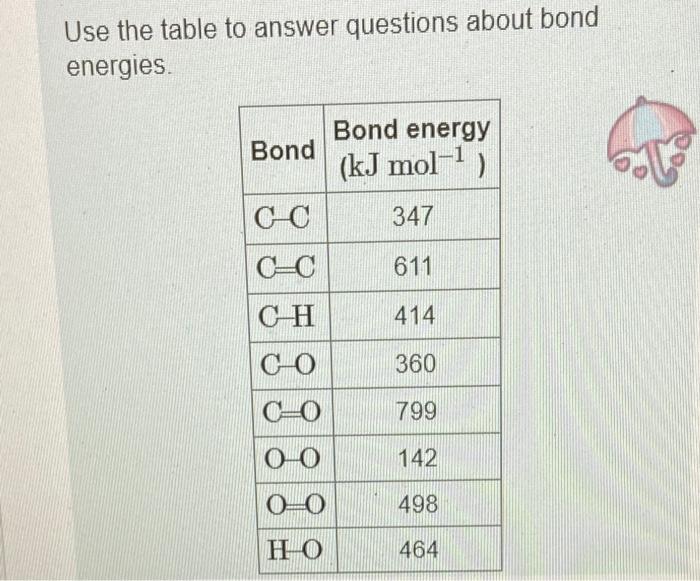
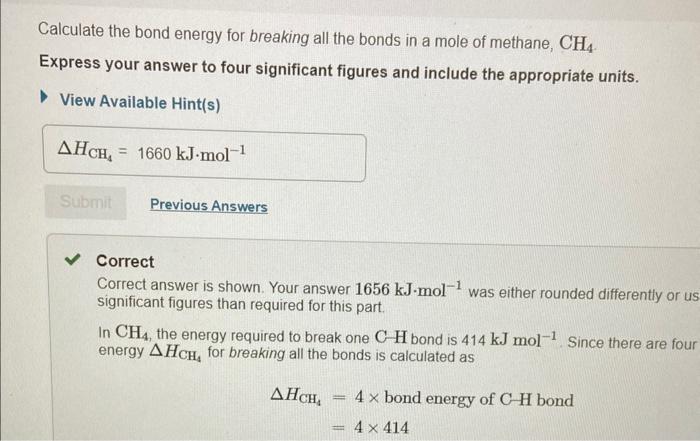
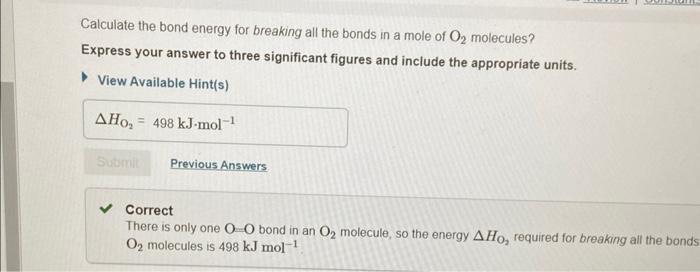
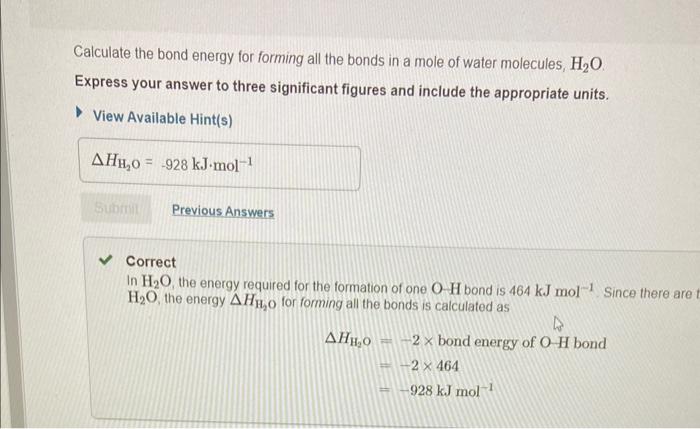
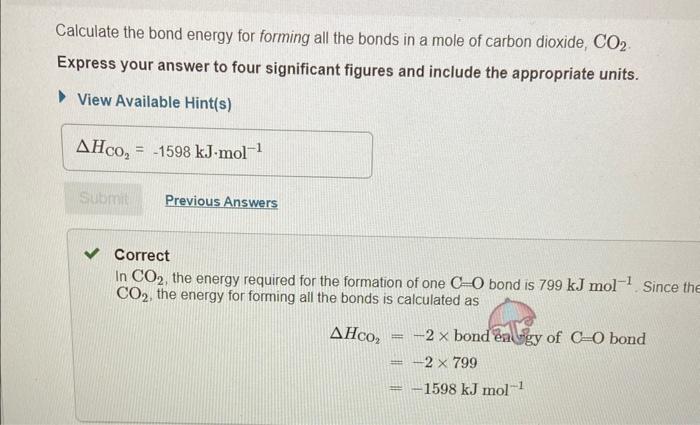
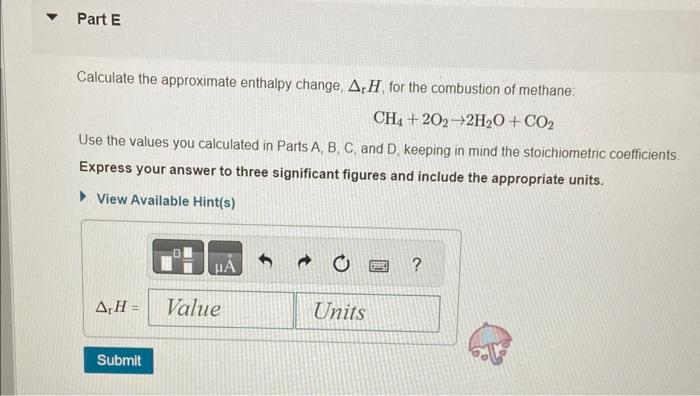
In chemical reactions, heat is converted into chemical energy (the potential energy stored in chemical bonds) or vice versa. Bond energy is the energy required to break one mole of the bond in the gas phase. Since it takes energy to break a bond, bond energies are always positive. Conversely, energy is released when bonds are formed. Thus, the enthalpy change for a reaction can be approximated from rH=(Hbreaking)+(Hforming) where H represents bond energies for the breaking (positive bond energy) or forming (negative bond energy) of a bond and Hr represents the overall enthalpy for the reaction. Use the table to answer questions about bond energies. Use the table to answer questions about bond energies. Calculate the bond energy for breaking all the bonds in a mole of methane, CH4. Express your answer to four significant figures and include the appropriate units. View Available Hint(s) Correct Correct answer is shown. Your answer 1656kJmol1 was either rounded differently or u significant figures than required for this part. In CH4, the energy required to break one CH bond is 414kJmol1. Since there are foul energy HCH4 for breaking all the bonds is calculated as HCH4=4bondenergyofCHbond=4414 Calculate the bond energy for breaking all the bonds in a mole of O2 molecules? Express your answer to three significant figures and include the appropriate units. View Available Hint(s) Correct There is only one OO bond in an O2 molecule, so the energy HO2, required for breaking all the bond: O2 molecules is 498kJmol1 Calculate the bond energy for forming all the bonds in a mole of water molecules, H2O. Express your answer to three significant figures and include the appropriate units. View Available Hint(s) Correct In H2O, the energy required for the formation of one OH bond is 464kJmol1 Since there are H2O, the energy HH2O for forming all the bonds is calculated as HH2O=2bondenergyofOHbond=2464=928kJmol1 Calculate the bond energy for forming all the bonds in a mole of carbon dioxide, CO2. Express your answer to four significant figures and include the appropriate units. View Available Hint(s) HCO2=1598kJmol1 Correct In CO2, the energy required for the formation of one C=O bond is 799kJmol1. Since th CO2, the energy for forming all the bonds is calculated as HCO2=2bondenigyofC=Obond=2799=1598kJmol1 Calculate the approximate enthalpy change, rH, for the combustion of methane: CH4+2O22H2O+CO2 Use the values you calculated in Parts A, B, C, and D, keeping in mind the stoichiometric coefficients. Express your answer to three significant figures and include the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


