Question: How do I find the intermediate rate constant (k') with [OCl-] 0.2754M and the the value of the overall rate constant (k)? I red dye
![How do I find the intermediate rate constant (k') with [OCl-] 0.2754M](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f84bcf61476_36766f84bcf03628.jpg)
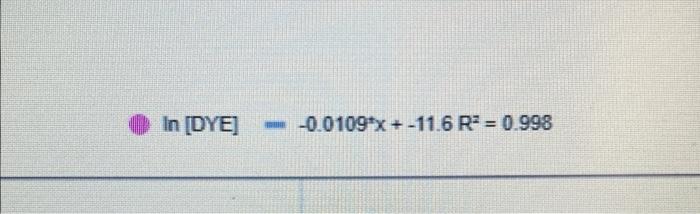
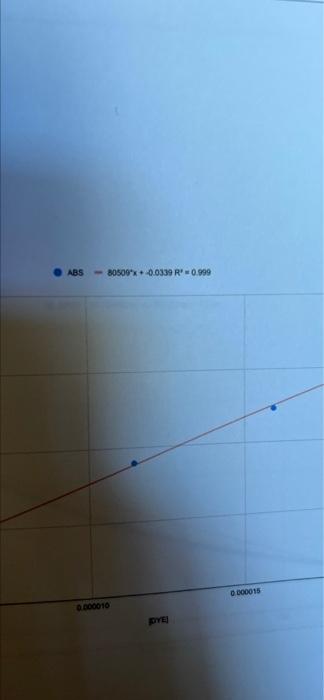
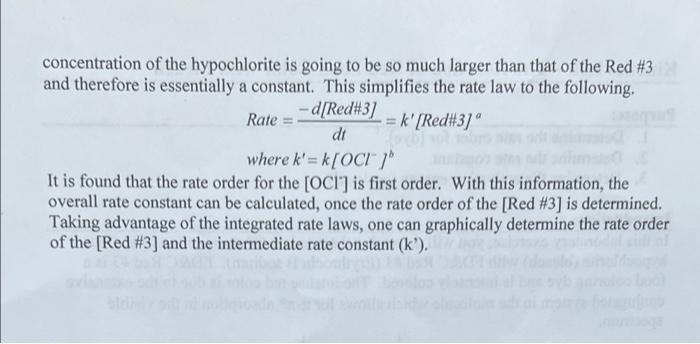
Calculations - Determination of Rate Orders - Part B: - What is the order of the chemical reaction in terms of [Red #3]: What is the value of the intermediate rate constant (k)? What is the value of the overall rate constant (k)? (Show your work.) 04 3 22 In [DYE] -0.01099x + -11.6 R = 0.998 ABS - 80509x + 0.0339 R' 0.999 D00015 000010 DYE concentration of the hypochlorite is going to be so much larger than that of the Red #3 and therefore is essentially a constant. This simplifies the rate law to the following. Rate = -d/Red#3] k' [Red#3]" dt where k'=k[OCI") It is found that the rate order for the [OCI') is first order. With this information, the overall rate constant can be calculated, once the rate order of the [Red #3] is determined. Taking advantage of the integrated rate laws, one can graphically determine the rate order of the [Red #3] and the intermediate rate constant (k)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


