Question: How do I get to mL? B asunjnstructurexom Question 2 2 pts Solute - a concentrated or solid substance to be dissolved or diluted Solvent
How do I get to mL?
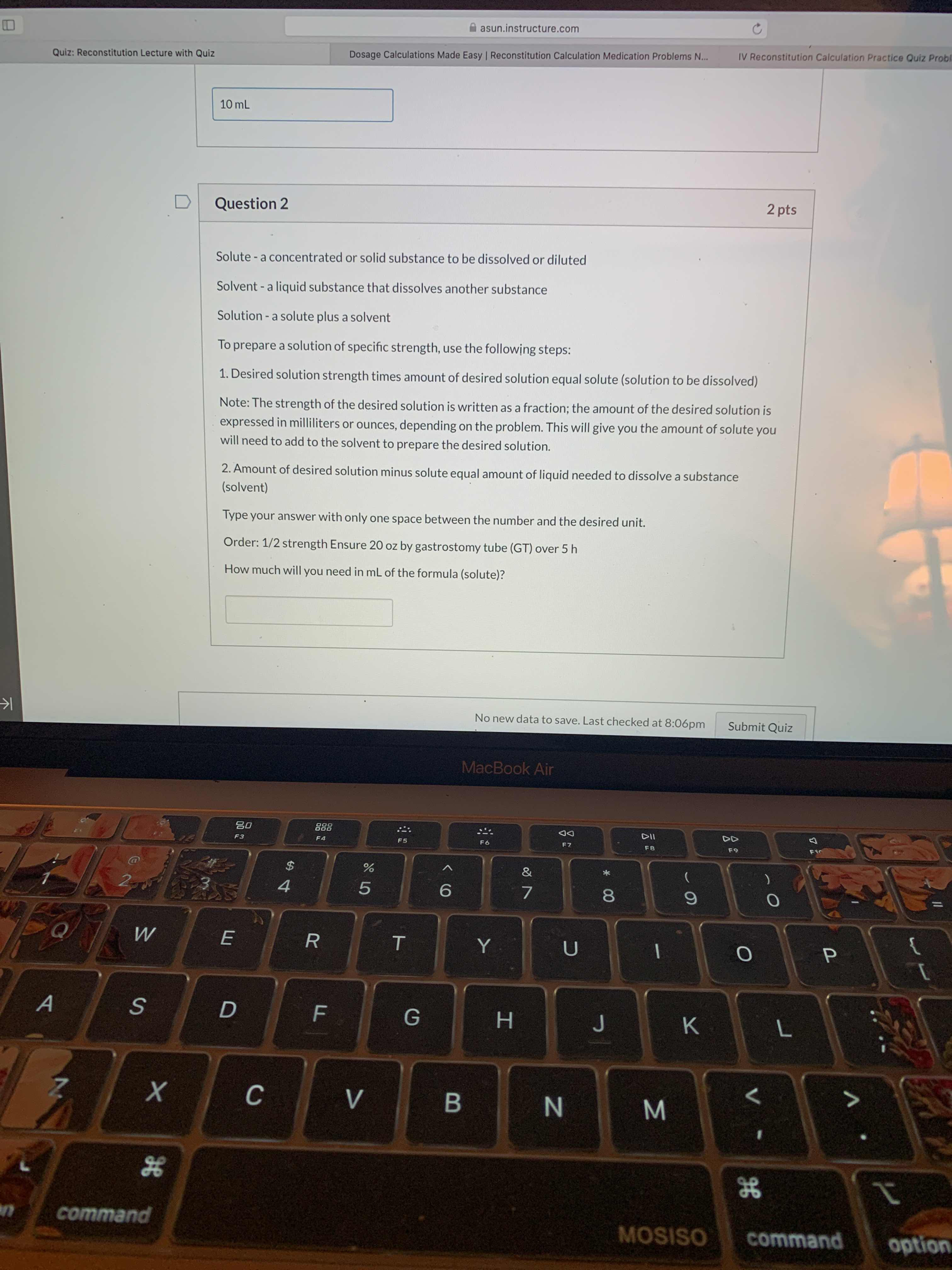
B asunjnstructurexom Question 2 2 pts Solute - a concentrated or solid substance to be dissolved or diluted Solvent - a liquid substance that dissolves another substance Solution - a solute plus a solvent To prepare a solution of specic strength. use the following steps: 1. Desired solution strength times amount of desired solution equal solute (solution to be dissolved) Note: The strength of the desired solution is written as a fraction: the amount of the desired solution is expressed in milliliters or ounces, depending on the problem. This will give you the amount of solute you will need to add to the solvent to prepare the desired solution. 2. Amount of desired solution minus solute equal amount of liquid needed to dissolve a substance (solvent) Type your answer with only one space between the number and the desired unit. Order: 1i2 strength Ensure 20 oz by gastrostomy tube (GT) over 5 h How much will you need in mL of the formula (solute)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


