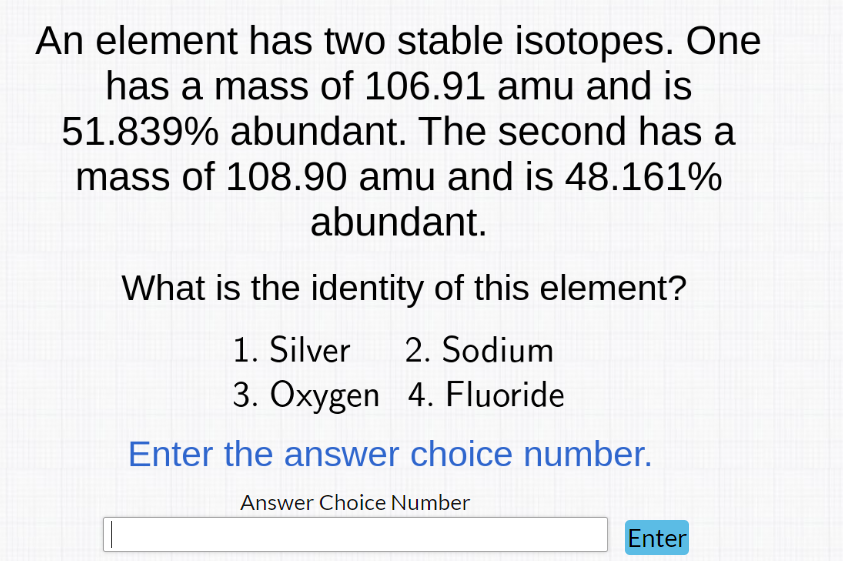
Question: How do I go about solving this? An element has two stable isotopes. One has a mass of 106.91 amu and is 51.839% abundant. The
 How do I go about solving this?
How do I go about solving this?
An element has two stable isotopes. One has a mass of 106.91 amu and is 51.839% abundant. The second has a mass of 108.90 amu and is 48.161% abundant. What is the identity of this element? 1. Silver 2. Sodium 3. Oxygen 4. Fluoride Enter the answer choice number. Answer Choice Number
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


