Question: How do I solve this? Liquid 4He boils at 4.2 K when the pressure of its vapor is 1 atmosphere. The latent heat of vaporizaton
How do I solve this?
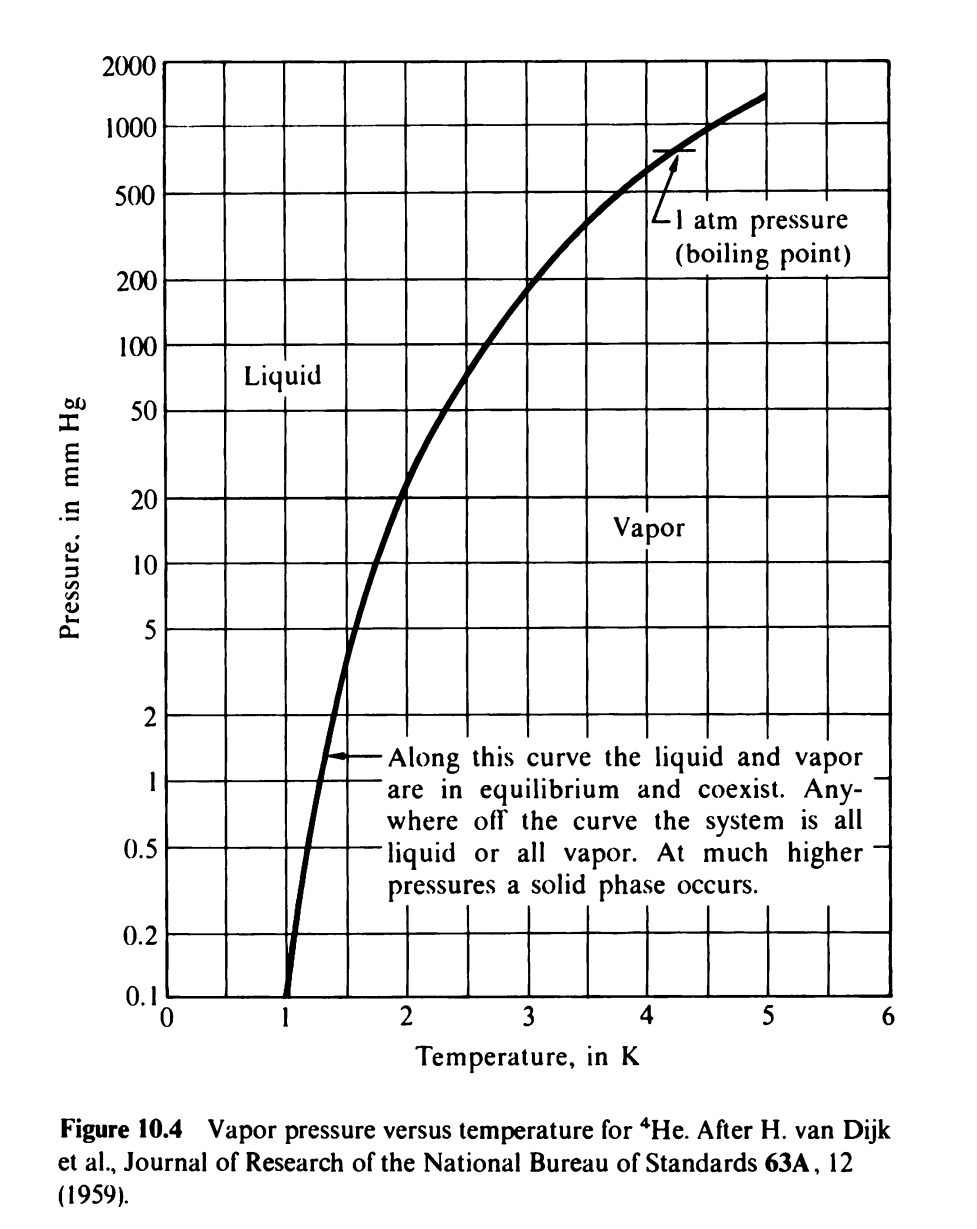
Liquid 4He boils at 4.2 K when the pressure of its vapor is 1 atmosphere. The latent heat of vaporizaton for a liquid is the energy required to convert 1 mole of liquid to a gas. For 4He the latent heat is 82 joules per mole and is approximately independent of temperature. We can lower the temperature of the 4He by pumping away the vapor (as we did in class). In addition most cryogenic systems have energy going into the liquid (heat) from outside. The system reaches equilibrium (i.e. it stops cooling) when the heat ow into the liquid is balanced by the energy taken away by evaporation. Assume that you can pump away the vapor at a rate of 36 m3 / hr (assume that the gas in the pump is at room temperature since it's warmed in the tube leading to the pump) and that there is heat leaking into the liquid at a rate of 0.1 Joules / s (because the container is not perfectly insulated). Assuming that the gaseous helium is an ideal gas, (i) nd the minimum vapor pressure that can be maintained (i.e. when you are in equilibrium). (ii) Using the curve in KK Fig. 10-4; estimate the minimum temperature that can be achieved. tm pressure (boiling point) Pressure. in mm Hg N o Along this curve the liquid and vapor are in equilibrium and coexist. Any- where off the curve the system is all liquid or all vapor. At much higher pressures a solid phase occurs. .l 0 0 1 2 3 4 5 6 Temperature. in K Figure 10.4 Vapor pressure versus temperature for 'He. After H. van Dijk el al., Journal of Research ofthe National Bureau of Standards 63A, 12 \"959)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


