Question: how do you do this The electrical potential, E, for the half-reaction 2MnO2(s)+2NH4+(aq)+2eMn2O3(s)+2NH3(aq)+H2O(l) is +/ V. (Record your three-digit answer in the numerical-response section on
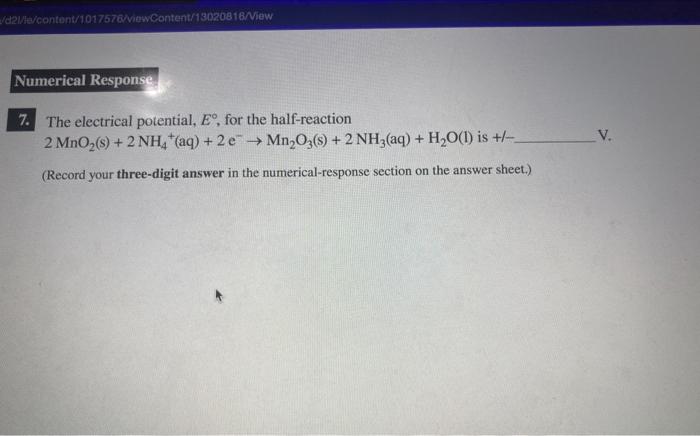
The electrical potential, E, for the half-reaction 2MnO2(s)+2NH4+(aq)+2eMn2O3(s)+2NH3(aq)+H2O(l) is +/ V. (Record your three-digit answer in the numerical-response section on the answer sheet.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


